108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

克唑替尼具有良好的疗效,除了通过下一代测序鉴定出 ROS1 重排的非小细胞肺癌伴随的突变
Authors Zeng L, Li Y, Xiao L, Xiong Y, Liu L, Jiang W, Heng J, Qu J, Yang N, Zhang Y
Received 14 June 2018
Accepted for publication 17 September 2018
Published 15 October 2018 Volume 2018:11 Pages 6937—6945
DOI https://doi.org/10.2147/OTT.S176273
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Introduction: Data of standard tyrosine kinase inhibitor (TKI) treatment outcome
in next-generation sequencing (NGS)-identified ROS1-rearranged non-small-cell
lung cancer (NSCLC) were rare. Thus, it is practical and necessary to evaluate
the efficacy and influential factors of crizotinib in real-world practice.
Patients and methods: A total of 1,466 NSCLC patients with positive
targeted NGS test results from September 2015 to January 2018 were enrolled in
this real-world retrospective study. Twenty-two patients had ROS1 rearrangement
detected by NGS. The efficacy and safety of crizotinib were evaluated.
Subgroups of concomitant mutations, brain metastasis, and fusion variants were
also analyzed.
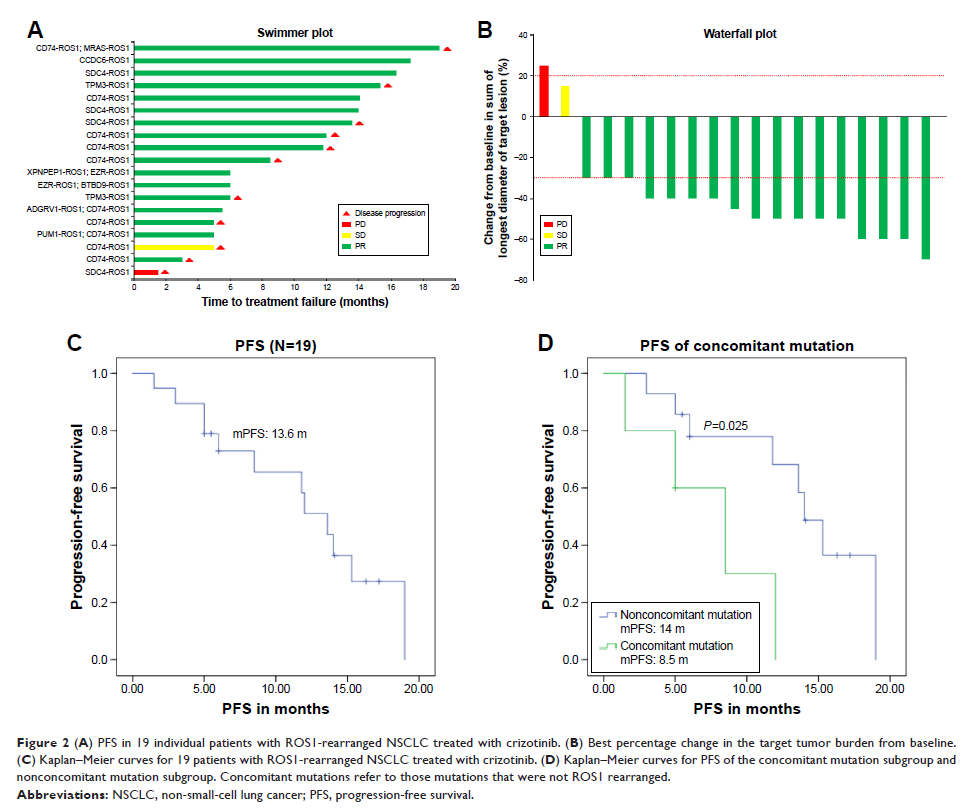
Results: Among all the patients, the occurrence rate of
ROS1 rearrangement was 1.5% (22 of 1,466). Ten ROS1 fusion partners were
detected, and the most common variant was CD74, which accounted for 50% (11 of
22). Five patients were found to carry dual ROS1 fusion partners, and 23% (5 of
22) of patients were detected with concomitant mutations, including
TP53&PIK3CA&mTOR mutation, TP53&CDKN2A mutation, TP53&BRCA2
mutation, ALK missense mutation (p.R311H), and MET amplification. Among 22
patients with ROS1-rearranged NSCLC, 20 patients were diagnosed at stage IV,
and 19 patients received crizotinib treatment. The average follow-up period was
16 months. The overall response rate (ORR) of crizotinib in unselected
crizotinib-treated patients was 89%, and the median progression-free survival
time (mPFS) was 13.6 months. It was shown that NSCLC patients with exclusive
ROS1 rearrangement had a longer PFS than those carrying concomitant mutations
(15.5 vs 8.5 months, P =0.0213). There were no newly occurring intolerant
adverse events in this study.
Conclusion: Crizotinib is highly effective in NGS-identified
ROS1-rearranged advanced NSCLC in real-word clinical practice, and the data are
consistent with previous clinical trials applying fluorescence in situ
hybridization/real-time PCR for ROS1 companion diagnosis. Concomitant mutations
may not be rare and may deteriorate the PFS of crizotinib in patients with
ROS1-rearranged NSCLC.
Keywords: ROS1,
next-generation sequencing, crizotinib, concomitant mutation, NSCLC, efficacy,
TP53, safety