108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

阿帕替尼治疗难治性恶性肿瘤的疗效和安全性:一个综述和全面分析
Authors Sun D, Hou H, Zhang C, Zhang X
Received 6 June 2018
Accepted for publication 10 August 2018
Published 5 October 2018 Volume 2018:11 Pages 6539—6554
DOI https://doi.org/10.2147/OTT.S176429
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Justinn Cochran
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev Srivastava
Background and purpose: Apatinib is a novel, oral, small-molecule tyrosine kinase inhibitor that targets VEGFR-2. Recent clinical trials have revealed its broad-spectrum anticancer effect. However, most recent studies of apatinib have involved single-arm studies with insufficient cases, different doses of drugs, and different incidences of adverse events (AEs), which has resulted in a lack of accurate measurement of the efficacy and safety of apatinib. Thus, we performed this meta-analysis to evaluate the efficacy and safety of apatinib.
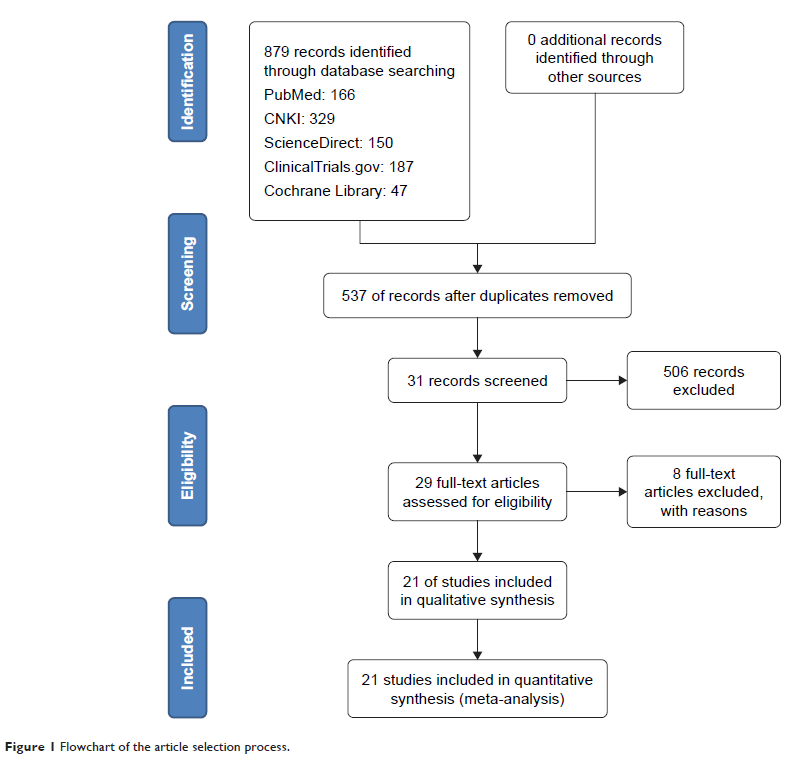
Methods: In total, 21 studies from five databases (PubMed, ScienceDirect, ClinicalTrials.gov, China National Knowledge Infrastructure [CNKI], and Cochrane Library) were included in this meta-analysis. All statistical analyses in this meta-analysis were performed using Stata 14.0 software. We used objective response rate (ORR) and disease control rate (DCR) to evaluate the efficacy of apatinib for five major types of solid tumors. Additionally, we used the total incidence of AEs and the incidence of the three most common grade 3–4 AEs to evaluate the safety of apatinib.
Results: The pooled results for the efficacy of apatinib in the treatment of different types of solid tumors revealed that patients treated with apatinib exhibited good disease control. In addition, it was likely that an increased dose of apatinib resulted in an increased ORR in lung and breast cancer and an increased DCR in liver and gastric cancer. Although AEs appeared in 84% of patients included in this meta-analysis, most of these AEs were of grades 1–2 and were well tolerated and controlled. The most common grade 3–4 AEs included hypertension, hand-foot syndrome, and proteinuria. Importantly, there were no significant differences in these grade 3–4 AEs with higher doses of apatinib.
Conclusion: Apatinib is a novel VEGFR-2 inhibitor with proven efficacy and safety for solid tumors. The meta-analysis reveals the broad-spectrum anticancer effect of apatinib.
Keywords: apatinib, solid tumors, objective response rate, disease control rate, adverse events