108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

负载利巴韦林的硒纳米粒通过抵抗 caspase-3 凋亡途径对 H1N1 流感病毒的感染进行限制
Authors Lin Z, Li Y, Gong G, Xia Y, Wang C, Chen Y, Hua L, Zhong J, Tang Y, Liu X, Zhu B
Received 19 June 2018
Accepted for publication 3 August 2018
Published 26 September 2018 Volume 2018:13 Pages 5787—5797
DOI https://doi.org/10.2147/IJN.S177658
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Yu Mi
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Introduction: Ribavirin (RBV) is a broad-spectrum antiviral drug. Selenium nanoparticles (SeNPs) attract much attention in the biomedical field and are used as carriers of drugs in current research studies. In this study, SeNPs were decorated by RBV, and the novel nanoparticle system was well characterized. Madin-Darby Canine Kidney cells were infected with H1N1 influenza virus before treatment with RBV, SeNPs, and SeNPs loaded with RBV (Se@RBV).
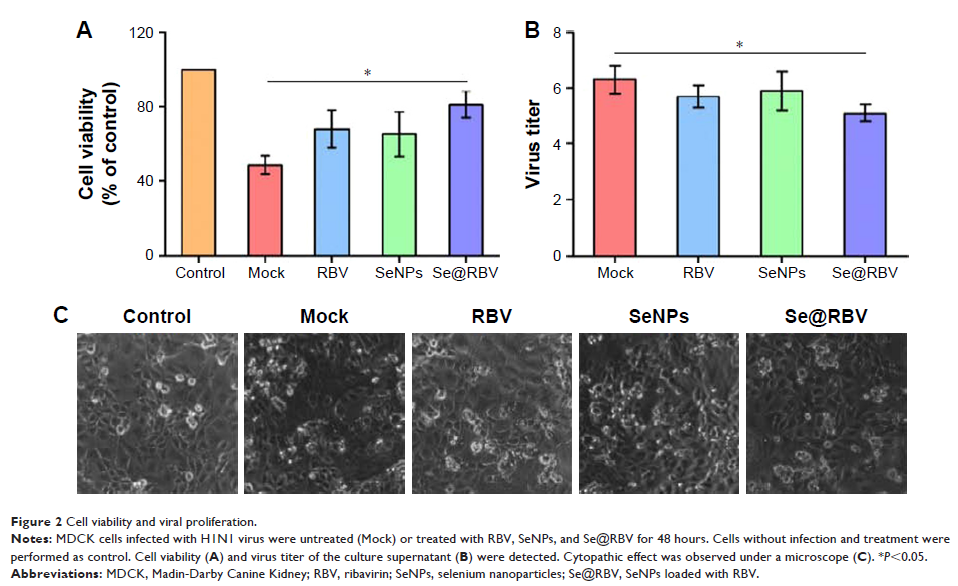
Methods and results: MTT assay showed that Se@RBV nanoparticles protect cells during H1N1 infection in vitro. Se@RBV depressed virus titer in the culture supernatant. Intracellular localization detection revealed that Se@RBV accumulated in lysosome and escaped to cytoplasm as time elapsed. Furthermore, activation of caspase-3 was resisted by Se@RBV. Expressions of proteins related to caspase-3, including cleaved poly-ADP-ribose polymerase, caspase-8, and Bax, were downregulated evidently after treatment with Se@RBV compared with the untreated infection group. In addition, phosphorylations of phosphorylated 38 (p38), JNK, and phosphorylated 53 (p53) were inhibited as well. In vivo experiments indicated that Se@RBV was found to prevent lung injury in H1N1-infected mice through hematoxylin and eosin staining. Tunel test of lung tissues present that DNA damage reached a high level but reduced substantially when treated with Se@RBV. Immunohistochemical test revealed an identical result with the in vitro experiment that activations of caspase-3 and proteins on the apoptosis pathway were restrained by Se@RBV treatment.
Conclusion: Taken together, this study elaborates that Se@RBV is a novel promising agent against H1N1 influenza virus infection.
Keywords: nanomaterials, H1N1 influenza virus, rivabirin, apoptosis, caspase-3