108552
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

先前的抗 CAF 可破坏 CAF 屏障并改善多西紫杉醇胶束在肿瘤中的积累
Authors Pang N, Li J, Sun A, Yang Z, Cheng S, Qi XR
Received 15 April 2018
Accepted for publication 16 July 2018
Published 4 October 2018 Volume 2018:13 Pages 5971—5990
DOI https://doi.org/10.2147/IJN.S171224
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Thiruganesh Ramasamy
Peer reviewer comments 3
Editor who approved publication: Dr Lei Yang
Background: Abnormal expression of stromal cells and extracellular matrix in
tumor stroma creates a tight barrier, leading to insufficient extravasation and
penetration of therapeutic agents. Cancer-associated fibroblasts (CAFs) take on
pivotal roles encouraging tumor progression.
Method: To surmount the refractoriness of stroma, we constructed a
multi-targeting combined scenario of anti-CAFs agent tranilast and antitumor
agent docetaxel micelles (DTX-Ms). Tranilast cut down crosstalk between tumor
cells and stromal cells, ameliorated the tumor microenvironment, and enhanced
the antiproliferation efficacy of DTX-Ms on cancer cells.
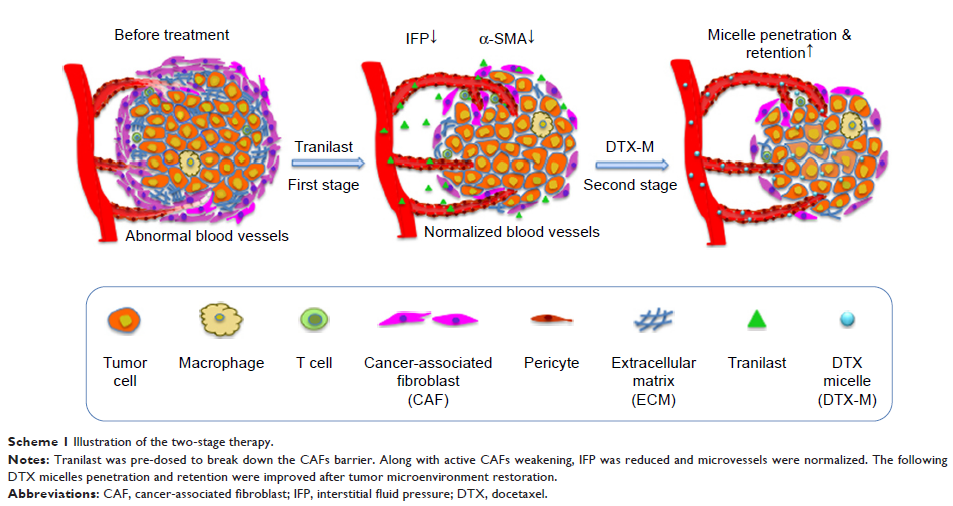
Results: Diverse experiments demonstrated that tranilast enhanced DTX-Ms’
antitumor effect in a two-stage pattern by CAFs ablation, tumor cell migration
blocking, and metastasis inhibition. Along with activated CAFs decreasing in
vivo, the two-stage therapy succeeded in reducing interstitial fluid pressure,
normalizing microvessels, improving micelles penetration and retention, and
inhibiting tumor growth and metastasis. Interestingly, tranilast alone failed
to inhibit tumor growth in vivo, and it could only be used as an adjuvant
medicine together with an antitumor agent.
Conclusion: Our proposed two-stage therapy offers a promising strategy to
enhance antitumor effects by breaking down CAFs barrier and increasing micellar
delivery efficiency.
Keywords: two-stage therapy, tumor microenvironment normalization,
cancer-associated fibroblasts, tranilast, stromal ablation