108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

IL-17 通过 NF-κB 诱导巨噬细胞形成 M2 样表型
Authors Shen J, Sun X, Pan B, Cao S, Cao J, Che D, Liu F, Zhang S, Yu Y
Received 22 May 2018
Accepted for publication 9 July 2018
Published 4 October 2018 Volume 2018:10 Pages 4217—4228
DOI https://doi.org/10.2147/CMAR.S174899
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr Luzhe Sun
Background: Tumor-associated macrophage (TAM) is emerging as one of the
important complications in cancer promotion. Interleukin-17 (IL-17), a potent
pro-inflammatory cytokine, plays an active role in promoting M2 macrophage
differentiation (TAMs are M2-like phenotypes). In this study, we aimed to
evaluate that IL-17 stimulates key phenotypic and functional signatures of M2
macrophages associated with cancer progression in non-small-cell lung cancer
(NSCLC) patients.
Patients and
methods: The markers and cytokines of M2
macrophages were detected in THP-1-derived macrophages and mouse peritoneal
macrophages treated with IL-17. The activation of nuclear factor kappa B
(NF-κB) and nuclear localization of p65 in IL-17-treated cells were
investigated. The BAY11-7082 inhibitor and the siRNA of p65 were used to block
the NF-κB activation. A total of 85 patients who underwent surgery for
histologically verified NSCLC were enrolled in this study. The expression of
IL-17 and M2 macrophage markers were assessed by immunostaining. Survivals were
estimated using the Kaplan–Meier method.
Results: The CD163 and CD206 cell surface markers and transforming growth
factor beta (TGF-β), vascular endothelial growth factor (VEGF) and IL-10 of M2
macrophages were significantly increased in IL-17-treated THP-1-derived
macrophages in a dose-dependent manner. IL-17 increased the mRNA levels of
Arginase I and Fizz1, the phosphorylation of IkBα and nuclear localization of
p65 (a subunit of NF-κB). The BAY11-7082 abrogated IL-17-induced CD206 and
CD163 expression, TGF-β, VEGF, IL-10, Arginase I and Fizz1 expression and p65
nuclear translocation. Further experiments showed that IL-17 induced the
expression of CD206, CD163, Arginase I, Fizz1 and Ym1 in mouse peritoneal
macrophages that were inhibited by siRNA of p65. The immunostaining experiments
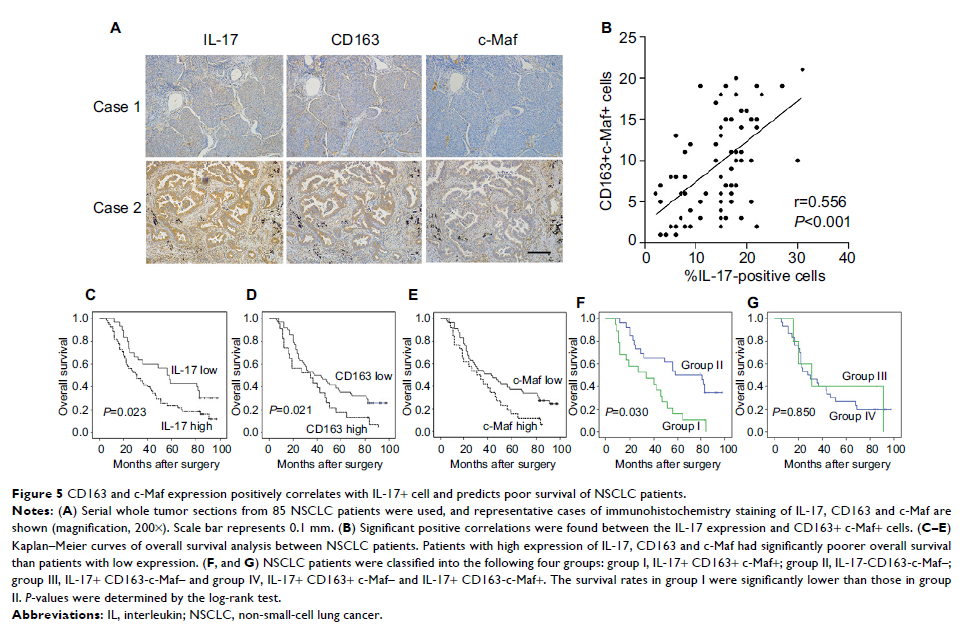
on human NSCLC tissues indicated that high IL-17 expression was significantly
correlated with CD163 and c-Maf. The intratumoral IL-17+ CD163+ c-Maf+ cells
were associated with NSCLC progression.
Conclusion: IL-17 stimulated macrophages to M2-like phenotypes via NF-κB
activation. IL-17 may be a potential therapeutic target for NSCLC.
Keywords: IL-17, M2 macrophages, NF-κB, non-small-cell lung cancer, survival