109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

罗格列酮 (Rosiglitazone) 可以使由吸烟诱导的肺气肿的金属蛋白酶/抗金属蛋白酶失衡减弱:细胞外信号调节激酶和 NFκB 信令的参与
Authors Hou G, Yin Y, Han D, Wang QY, Kang J
Published Date April 2015 Volume 2015:10(1) Pages 715—724
DOI http://dx.doi.org/10.2147/COPD.S77514
Received 14 November 2014, Accepted 21 January 2015, Published 7 April 2015
Objective: We
investigated how rosiglitazone attenuated cigarette smoke (CS)-induced
emphysema in a rat model. In particular, we focused on its possible effects on
the imbalance between metalloprotease (MMP) and anti-MMP activity,
mitogen-activated protein kinase (MAPK) phosphorylation, and nuclear factor
kappa-light-chain-enhancer of activated B cell (NFκB) signaling pathway
over-activation.
Methods: A total of 36 Wistar
rats were divided into three groups (n=12 each): animals were exposed to CS for
12 weeks in the absence (the CS group) or presence of 30 mg/kg rosiglitazone
(the rosiglitazone-CS [RCS] group); a control group was treated with the
rosiglitazone vehicle only, without any CS exposure. Histopathology of lung
tissue in all groups was evaluated to grade severity of the disease. Expression
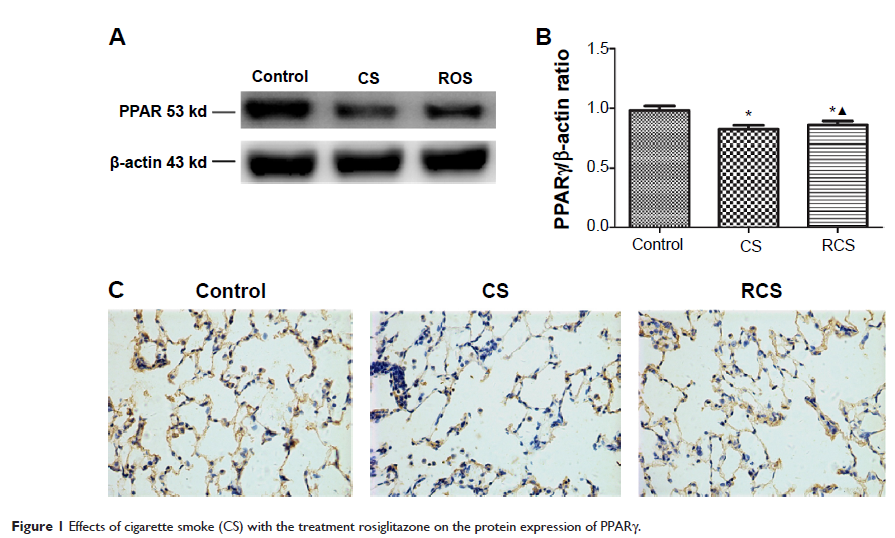
levels of peroxisome proliferator-activated receptor γ (PPARγ), MMP2, and MMP9
in lung tissue were determined and compared using Western blotting and
immunohistochemistry. Activation of MAPKs, NFκB, and the nuclear factor of
kappa light polypeptide gene enhancer in B-cell inhibitor, alpha (IκBα)
phosphorylation in lung tissue was examined by Western blotting.
Results: Emphysema-related
pathology, based on inter-alveolar wall distance and alveolar density, was less
severe in the RCS group than in the CS group. Compared with the CS group,
levels of PPARγ were higher in the RCS group, and levels of MMP2 and MMP9
proteins were lower in the RCS rats. Levels of activated MAPKs and NFκB were
also lower, while the IκBαphosphorylation was increased in the lung tissue of RCS
rats.
Conclusion: Our findings
suggest that oral administration of rosiglitazone attenuates the
metalloprotease activity induced by CS, and the underlying mechanism might
involve the activation of signaling pathways dependent on MAPKs or NFκB. Our
results further suggest that PPARγ contributes to the pathogenesis of emphysema
as well as airway inflammation induced by CS.
Keywords: emphysema, chronic
obstructive pulmonary disease, matrix metalloprotease9, matrix
metalloprotease2, PPAR, NFκB