108552
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

一种新型的负载三氧化二砷的 RGDyC/PEG 共修饰的 PAMAM 树枝状化合物,作为胶质瘤靶向递送系统
Authors Lu Y, Han S, Zheng H, Ma R, Ping Y, Zou J, Tang HX, Zhang Y, Xu X, Li F
Received 26 May 2018
Accepted for publication 2 August 2018
Published 2 October 2018 Volume 2018:13 Pages 5937—5952
DOI https://doi.org/10.2147/IJN.S175418
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: The Traditional Chinese Medicine, arsenic trioxide (ATO, As2O3) could inhibit
growth and induce apoptosis in a variety of solid tumor cells, but it is
severely limited in the treatment of glioma due to its poor BBB penetration and
nonspecifcity distribution in vivo.
Purpose: The objective of this study was encapsulating
ATO in the modified PAMAM dendrimers to solve the problem that the poor
antitumor effect of ATO to glioma, which provide a novel angle for the study of
glioma treatment.
Methods: The targeting drug carrier (RGDyC-mPEG-PAMAM)
was synthesized based on Arg-Gly-Asp (RGDyC) and αvβ3 integrin targeting ligand,
and conjugated to PEGylated fifth generation polyamidoamine dendrimer
(mPEG-PAMAM). It was characterized by nuclear magnetic resonance, fourier
transform infrared spectra, Nano-particle size-zeta potential analyzer, etc.
The in vitro release characteristics were studied by dialysis bag method. MTT
assay was used to investigate the cytotoxicity of carriers and the antitumor
effect of ATO formulation. In vitro blood-brain barrier (BBB) and C6 cell
co-culture models were established to investigate the inhibitory effect of
different ATO formulation after transporting across BBB. Pharmacokinetic and
antitumor efficacy studies were investigated in an orthotopic murine model of
C6 glioma.
Results: The prepared RGDyC-mPEG-PAMAM was characterized
for spherical dendrites, comparable size (21.60±6.81 nm), and zeta potential
(5.36±0.22 mV). In vitro release showed that more ATO was released from
RGDyC-mPEG-PAMAM/ATO (79.5%) at pH 5.5 than that of pH 7.4, during 48 hours.
The cytotoxicity of PEG-modified carriers was lower than that of the naked
PAMAM on both human brain microvascular endothelial cells and C6 cells. In in
vitro BBB model, modification of RGDyC heightened the cytotoxicity of ATO
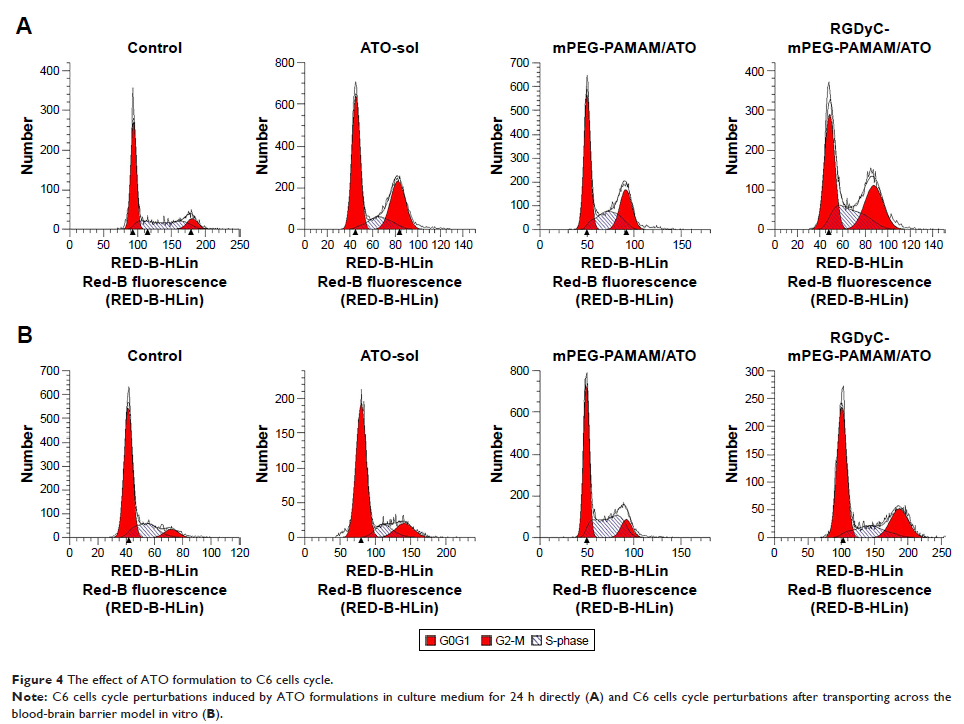
loaded on PAMAM, due to an increased uptake by C6 cells. The results of cell
cycle and apoptosis analysis revealed that RGDyC-mPEG-PAMAM/ATO arrested the
cell cycle in G2-M and exhibited threefold increase in percentage of apoptosis
to that in the PEG-PAMAM/ATO group. Compared with ATO-sol group, both
RGDyC-mPEG-PAMAM/ATO and mPEG-PAMAM/ATO groups prolonged the half-life time,
increased area under the curve, and improved antitumor effect, significantly.
While the tumor volume inhibitory of RGDyC-mPEG-PAMAM/ATO was 61.46±12.26%, it
was approximately fourfold higher than the ATO-sol group, and twofold to the
mPEG-PAMAM/ATO group.
Conclusion: In this report, RGDyC-mPEG-PAMAM could enhance
the antitumor of ATO to glioma, it provides a desirable strategy for targeted
therapy of glioma.
Keywords: arsenic
trioxide, blood-brain barrier, RGDyC, PEG co-modified, glioma targeting
delivery, PAMAM dendrimer