108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

程序性死亡配体 1 在人肝内胆管细胞癌中的表达及与预后和 CD8+ T 细胞免疫应答的关系
Authors Zhu Y, Wang XY, Zhang Y, Xu D, Dong J, Zhang Z, Yi CH, Jia HL, Yang X
Received 1 May 2018
Accepted for publication 22 June 2018
Published 2 October 2018 Volume 2018:10 Pages 4113—4123
DOI https://doi.org/10.2147/CMAR.S172719
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Professor Nakshatri
Background: Agents targeting the programmed death ligand 1 (PD-L1)/programmed death
receptor 1 immune checkpoint exhibited promising clinical outcomes in a variety
of malignant tumors, including intrahepatic cholangiocarcinoma (ICC). However,
the relationship between PD-L1 expression and CD8+ T-cell immune responses is not well defined in ICC.
Patients and
methods: We investigated PD-L1
expression immunohistochemistry in formalin-fixed, paraffin-embedded tissues
from 192 ICC patients undergoing curative resection and correlated our results
with the clinicopathologic features and prognosis. We also quantified CD8+ T-cell infiltration in ICC specimens and evaluated the
relationship between PD-L1 expression and CD8+ T-cell infiltration. After incubating human ICC cell lines
(HCCC9810 and RBE) with interferon (IFN)-γ, we measured the PD-L1
expression of these ICC cells by Western blot and flow cytometry.
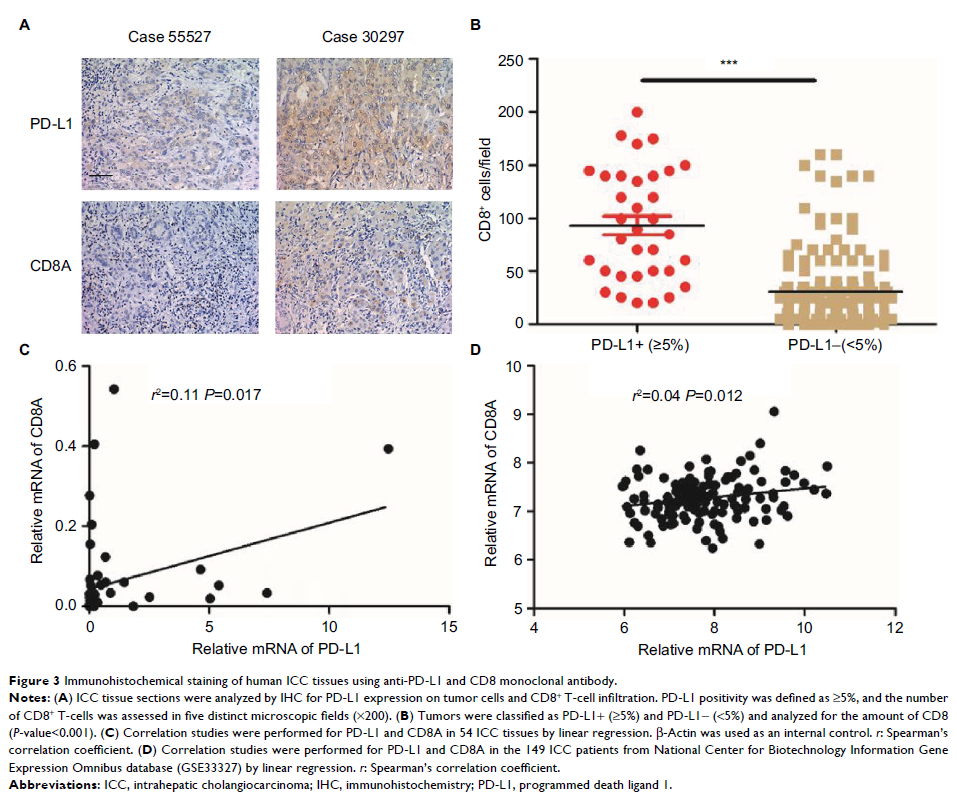
Results: Only 34 patients (17.7%) showed ≥5% membranous PD-L1 expression on
tumor cells, and tumoral PD-L1 overexpression (≥5%) was significantly
associated with superior overall survival (P =0.012) and
disease-free survival (P =0.018). A
significant positive association was found between PD-L1 expression and the
presence of CD8+ T-cells. In fresh
frozen ICC specimens, IFN-γ was found to be significantly correlated with PD-L1
and CD8A gene expression, as evaluated by reverse transcription-polymerase
chain reaction. Moreover, stimulation of the HCCC9810 and RBE cells with
recombinant IFN-γ, secreted by CD8+ T-cells rapidly induced PD-L1 upregulation in these cell lines in
vitro.
Conclusion: Tumor PD-L1 overexpression is mainly stimulated by activated CD8+ T-cells pre-existing in the ICC microenvironment, and PD-L1 is a
favorable prognostic factor for the patients. These observations suggest that
anti-PD-L1/programmed death receptor 1 therapy may benefit ICC patients with
tumor cell PD-L1 expression and the presence of CD8+ T-cells.
Keywords: tumor microenvironment, adaptive immune resistance, PD-L1, CD8+ T-cell, IFN-γ