108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

瑞波非尼治疗晚期实体瘤的不良事件危险性:对随机对照试验的一项综合分析
Authors Yin X, Yin Y, Shen C, Chen H, Wang J, Cai Z, Chen Z, Zhang B
Received 11 November 2017
Accepted for publication 12 August 2018
Published 2 October 2018 Volume 2018:11 Pages 6405—6414
DOI https://doi.org/10.2147/OTT.S156760
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Background: Regorafenib is a novel multikinase inhibitor (MKI) approved for
use in the treatment of metastatic colorectal cancer (CRC),
treatment-refractory gastrointestinal stromal tumors, and other solid tumor
malignancies. However, the adverse events (AEs) associated with regorafenib
have not been systematically investigated. Hence, we performed a meta-analysis
to identify AEs associated with regorafenib in patients with advanced solid
tumors.
Methods: The databases of PubMed, MEDLINE, and Embase and
abstracts presented in American Society of Clinical Oncology annual meetings
were searched for relevant publications from January 2004 to September 2017.
Eligible studies were limited to prospective randomized controlled trials
(RCTs) that evaluate the use of regorafenib in patients with advanced solid
tumors. Incidence, relative risk (RR), and 95% CIs were calculated using a
random or fixed effects model on the basis of the heterogeneity of the included
studies.
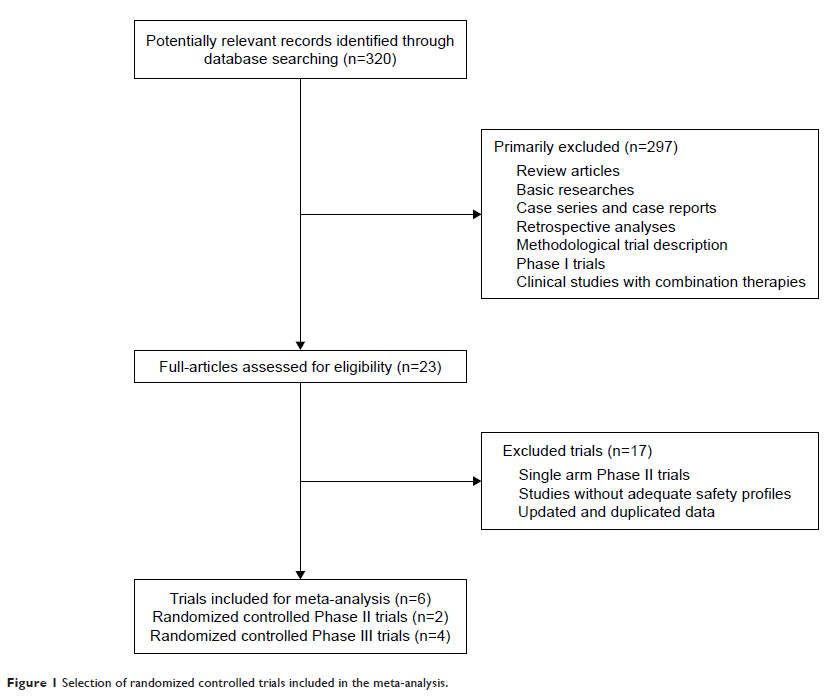
Results: A total of 2,065 patients from six RCTs were
included, and 1,340 of them received regorafenib and 725 received a placebo.
Sixteen all-grade AEs and 15 high-grade AEs were investigated for their
association with regorafenib. Results showed that hand–foot skin reaction
(HFSR; 54%), diarrhea (33%), fatigue (32%), hypertension (31%), oral mucositis
(28%), and anorexia (23%) were the most frequent clinical AEs. The most common
high-grade (grade, $3) AEs were HFSR (16%), hypertension (13%), fatigue (6%),
increased aspartate aminotransferase (AST; 6%), and hypophosphatemia (6%). Pooled
RR showed that the use of regorafenib was associated with an increased risk of
developing AEs. Subgroup analysis based on the prior MKI treatment showed that
prior MKI treatment was associated with an increased incidence of all-grade
anorexia (P =0.03) and a reduced incidence of
high-grade increased AST (P =0.04). However,
subgroup analysis based on the tumor type showed that no significant
differences were found when comparing the RR of all-grade and high-grade AEs in
patients with CRC or non-CRC.
Conclusion: The meta-analysis systematically investigated
regorafenib-associated AEs. Knowledge of these AEs is essential for minimizing
treatment-related toxicities and improving clinical outcomes.
Keywords: regorafenib,
adverse event, AE, safety, multikinase inhibitor, meta-analysis