108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
分子靶向药物治疗晚期肝细胞癌的致命不良事件:对随机对照试验的一项综合分析
Authors Li X, Wan J, Wu Z, Tu J, Hu Y, Wu S, Lou L
Received 8 September 2017
Accepted for publication 10 April 2018
Published 18 September 2018 Volume 2018:12 Pages 3043—3049
DOI https://doi.org/10.2147/DDDT.S151241
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Aims: Concerns have
increased about the risk of fatal adverse events (FAEs) associated with
molecular targeted agents (MTAs) in the treatment of advanced hepatocellular
carcinoma (HCC). The purpose of this study is to investigate the overall
incidence and risk of FAEs in advanced HCC with administration of MTAs by using
a meta-analysis of available clinical trials.
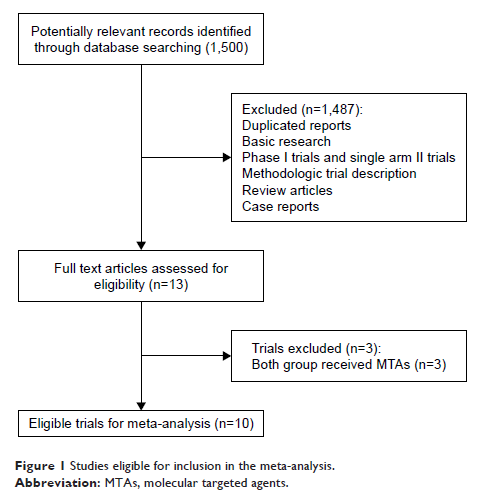
Materials and methods: Electronic databases were searched for relevant articles before March 2017. Eligible studies were selected according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. Pooled incidence, Peto ORs and 95% CIs were calculated according to the heterogeneity of selected studies.
Results: A total of 4,716 HCC participants from 10 randomized controlled trials (RCTs) were finally considered for this meta-analysis. The pooled incidence of death due to MTAs was 2.1% (95% CI 1.6%–2.8%) with a Peto OR of 1.79 (95% CI 1.07–3.01; p =0.027) in comparison with controlled groups. Subgroup analysis according to biological agents showed that brivanib treatment in HCC patients significantly increased the risk of developing FAEs (Peto OR 3.97; 95% CI 1.17–13.51; p =0.028) but not for sorafenib (Peto OR 1.78; 95% CI 0.54–5.89; p =0.34) and other MTAs (Peto OR 1.43; 95% CI 0.75–2.76; p =0.28). Sensitive analysis showed that the pooled results were influenced by removing each single trial. The most common causes of FAEs were hepatic failure (22.2%) and hemorrhage (13.3%), respectively.
Conclusion: Clinicians should be aware of the risks of FAEs during the administration of MTAs in advanced HCC patients, especially for patients with abnormal liver function. However, the use of sorafenib remains justified in its approved indications due to their potential survival benefits and limited toxicities.
Keywords: liver cancer, clinical trials, novel molecular agents
Materials and methods: Electronic databases were searched for relevant articles before March 2017. Eligible studies were selected according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. Pooled incidence, Peto ORs and 95% CIs were calculated according to the heterogeneity of selected studies.
Results: A total of 4,716 HCC participants from 10 randomized controlled trials (RCTs) were finally considered for this meta-analysis. The pooled incidence of death due to MTAs was 2.1% (95% CI 1.6%–2.8%) with a Peto OR of 1.79 (95% CI 1.07–3.01; p =0.027) in comparison with controlled groups. Subgroup analysis according to biological agents showed that brivanib treatment in HCC patients significantly increased the risk of developing FAEs (Peto OR 3.97; 95% CI 1.17–13.51; p =0.028) but not for sorafenib (Peto OR 1.78; 95% CI 0.54–5.89; p =0.34) and other MTAs (Peto OR 1.43; 95% CI 0.75–2.76; p =0.28). Sensitive analysis showed that the pooled results were influenced by removing each single trial. The most common causes of FAEs were hepatic failure (22.2%) and hemorrhage (13.3%), respectively.
Conclusion: Clinicians should be aware of the risks of FAEs during the administration of MTAs in advanced HCC patients, especially for patients with abnormal liver function. However, the use of sorafenib remains justified in its approved indications due to their potential survival benefits and limited toxicities.
Keywords: liver cancer, clinical trials, novel molecular agents