109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

将加载了 GEM 并用西妥昔单抗改性的磁性白蛋白纳米球用于同时定位、磁共振成像及对胰腺癌细胞的双重靶向热化疗
Authors Wang L, An Y, Yuan C, Zhang H, Liang C, Ding F, Gao Q, Zhang D
Published Date March 2015 Volume 2015:10 Pages 2507—2519
DOI http://dx.doi.org/10.2147/IJN.S77642
Received 17 November 2014, Accepted 16 March 2015, Published 30 March 2015
Background: Targeted
delivery is a promising strategy to improve the diagnostic imaging and
therapeutic effect of cancers. In this paper, novel cetuximab
(C225)-conjugated, gemcitabine (GEM)-containing magnetic albumin nanospheres
(C225-GEM/MANs) were fabricated and applied as a theranostic nanocarrier to
conduct simultaneous targeting, magnetic resonance imaging (MRI), and
double-targeted thermochemotherapy against pancreatic cancer cells.
Methods: Fe3O4 nanoparticles (NPs) and GEM co-loaded albumin nanospheres (GEM/MANs) were
prepared, and then C225 was further conjugated to synthesize C225-GEM/MANs.
Their morphology, mean particle size, GEM encapsulation ratio, specific
cell-binding ability, and thermal dynamic profiles were characterized. The
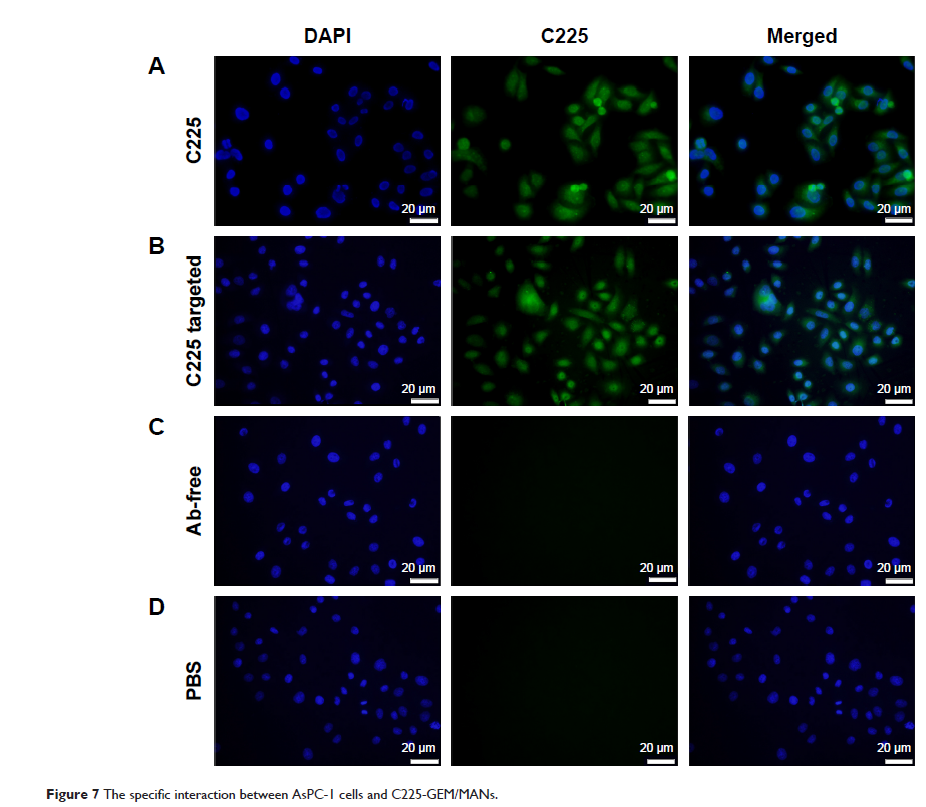
effects of discriminating different EGFR-expressing pancreatic cancer cells
(AsPC-1 and MIA PaCa-2) and monitoring cellular targeting effects were assessed
by targeted MRI. Lastly, the antitumor efficiency of
double/C225/magnetic-targeted and nontargeted thermochemotherapy was compared
with chemotherapy alone using 3-(4,
5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and flow cytometry
(FCM) assay.
Results: When treated with
targeted nanospheres, AsPC-1 cells showed a significantly less intense MRI T2
signal than MIA PaCa-2 cells, while both cells had similar signal strength when
incubated with nontargeted nanospheres. T2 signal intensity was significantly
lower when magnetic and C225 targeting were combined, rather than used alone.
The inhibitory and apoptotic rates of each thermochemotherapy group were
significantly higher than those of the chemotherapy-alone groups. Additionally,
both MTT and FCM analysis verified that double-targeted thermochemotherapy had
the highest targeted killing efficiency among all groups.
Conclusion: The C225-GEM/MANs
can distinguish various EGFR-expressing live pancreatic cancer cells, monitor
diverse cellular targeting effects using targeted MRI imaging, and efficiently
mediate double-targeted thermochemotherapy against pancreatic cancer cells.
Keywords: gemcitabine, Fe3O4 nanoparticles, theranostic nanocarrier