108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

用于治疗溃疡性结肠炎的 pH 敏感性布地奈德纳米粒的制备和表征
Authors Zhou H, Qian H
Received 10 April 2018
Accepted for publication 17 May 2018
Published 22 August 2018 Volume 2018:12 Pages 2601—2609
DOI https://doi.org/10.2147/DDDT.S170676
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Objective: The aim of this study was to develop pH sensitive nanoparticles of budesonide for the treatment of ulcerative colitis.
Methods: The NPs system was characterized by the transmission electron microscopy (TEM), particle size, drug loading and encapsulation efficiency. In addition, in vitro drug release properties and pharmacokinetics were also investigated in detail. The optimized formulation was examined for its in-vivo targeting potential using 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis in a rat model.
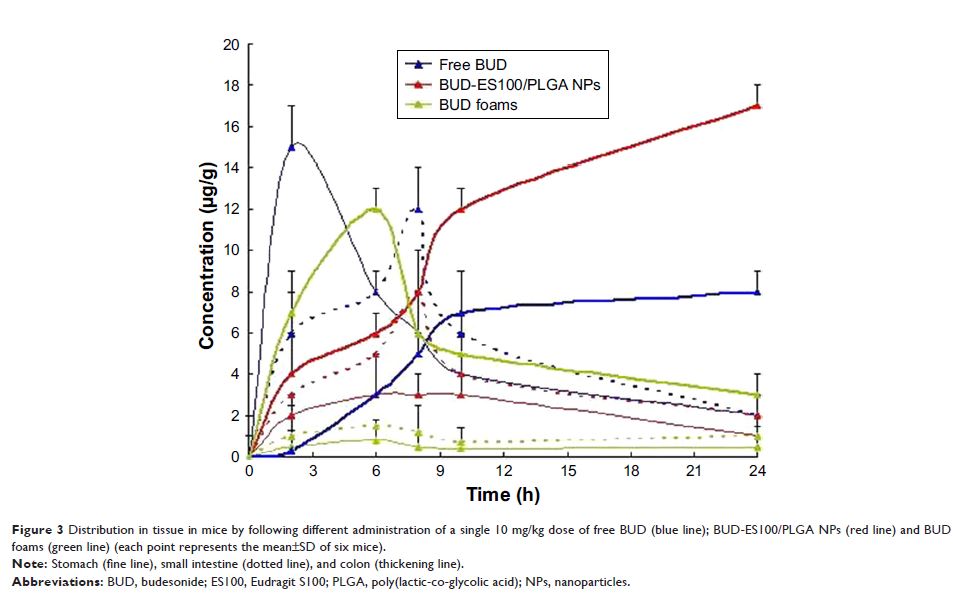
Results: Dynamic light-scattering results showed that the particle size of budesonide-Eudragit S100/poly(lactic-co-glycolic acid) nanoparticles was around 110.5 nm, with a polydispersity index of 0.098. Transmission electron microscopy images showed that BUD-ES100/PLGA NPs were spherical with uniform size and relatively smooth surfaces. In vitro release showed that BUD-ES100/PLGA NPs required minimal release of drugs during its transit in the stomach and the upper small intestine to ensure that a maximum dose reached the colon. After the pharmacodynamic treatment, the myeloperoxidase value of BUD-ES100/PLGA NPs was close to the normal group. The histopathological examination of rectum showed that no sign of damages such as epithelial necrosis and sloughing epithelial cells was detected.
Conclusion: Our findings suggested that BUD-ES100/PLGA NPs were a promising alternative to single pH-dependent systems for colitis therapy.
Keywords: budesonide, nanoparticles, ES100/PLGA, in vitro release, pharmacodynamic