108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

金黄色葡萄球菌 α-溶血素中的两个残基与溶血和自组装有关
Authors Du Y, Liu L, Zhang C, Zhang Y
Received 9 March 2018
Accepted for publication 12 May 2018
Published 21 August 2018 Volume 2018:11 Pages 1271—1274
DOI https://doi.org/10.2147/IDR.S167779
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
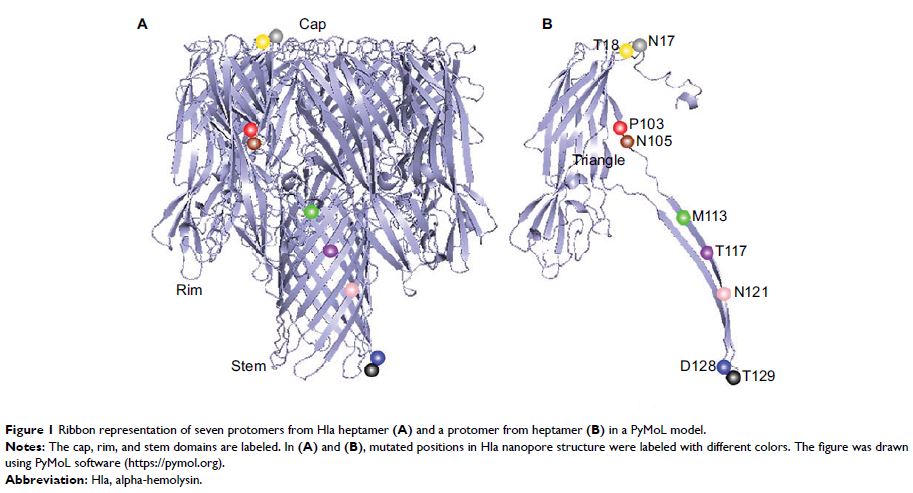
Abstract: Staphylococcus aureus is becoming increasingly intractable because of its ability to acquire antimicrobial resistance and secrete numerous virulence factors that can exacerbate inflammation. Alpha-hemolysin (Hla) is a pore-forming virulence factor produced by S. aureus that can self-assemble into heptameric mushroom-structured pores in target cell membranes, leading to cell lysis and death. In the present study, we sought to better understand the mechanism underlying hemolysis and the oligomerization of Hla by creating nine mutants with single amino acid changes in different positions of the Hla protein: N17C, T18C, P103C, N105C, M113C, T117C, N121C, D128C, and T129C. The results showed that the P103C and N105C mutations, which are located in the triangle region, significantly diminished hemolysis and heptamer formation when compared with the wild-type Hla protein. This suggests that the P103 and N105 residues play key roles in the assembly of the Hla pore. These results improve our understanding of the mechanism underlying the pore-forming ability of Hla.
Keywords: α-hemolysin, hemolysis, heptamer oligomers, cysteine mutants, assembly