108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

应用基于超临界流体技术的可吸入 siRNA 纳米粒子修饰的多孔微粒克服多药耐药性
Authors Xu PY, Kankala RK, Pan YJ, Yuan H, Wang SB, Chen AZ
Received 27 March 2018
Accepted for publication 19 June 2018
Published 15 August 2018 Volume 2018:13 Pages 4685—4698
DOI https://doi.org/10.2147/IJN.S169399
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Farooq Shiekh
Peer reviewer comments 5
Editor who approved publication: Dr Linlin Sun
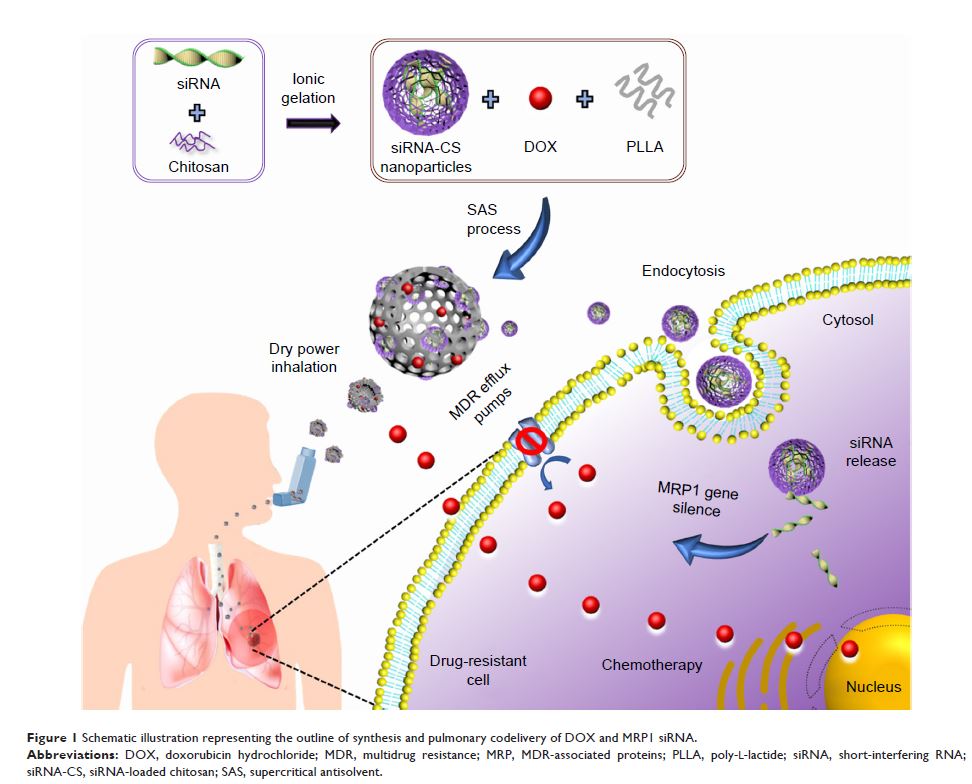
Background: In recent times, the co-delivery therapeutics have garnered enormous interest from researchers in the treatment of cancers with multidrug resistance (MDR) due to their efficient delivery of multiple agents, which result in synergistic effects and capable of overcoming all the obstacles of MDR in cancer. However, an efficient delivery platform is required for the conveyance of diverse agents that can successfully devastate MDR in cancer.
Methods: Initially, short-interfering RNA-loaded chitosan (siRNA-CS) nanoparticles were synthesized using the ionic gelation method. Further, the siRNA-CS nanoparticles and doxorubicin hydrochloride (DOX) were co-loaded in poly-L-lactide porous microparticles (PLLA PMs) (nano-embedded porous microparticles, [NEPMs]) by the supercritical anti-solvent (SAS) process.
Results and discussion: The NEPM formulation exhibited an excellent aerodynamic performance and sustained release of DOX, which displayed higher anticancer efficacy in drug-resistant cells (human small cell lung cancer, H69AR cell line) than those treated with either free DOX and DOX-PLLA PMs due to the siRNA from CS nanoparticles silenced the MDR gene to DOX therapy.
Conclusion: This eco-friendly process provides a convenient way to fabricate such innovative NEPMs co-loaded with a chemotherapeutic agent and a gene, which can devastate MDR in cancer through the co-delivery system.
Keywords: pulmonary delivery, short-interfering RNA, multidrug resistance, doxorubicin, supercritical carbon dioxide