108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

塞来昔布治疗晚期非小细胞肺癌的优势的系统评价和荟萃分析
Authors Yi L, Zhang W, Zhang H, Shen J, Zou J, Luo P, Zhang J
Received 29 March 2018
Accepted for publication 22 May 2018
Published 7 August 2018 Volume 2018:12 Pages 2455—2466
DOI https://doi.org/10.2147/DDDT.S169627
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Background: The clinical benefit of a selective cyclooxygenase-2 inhibitor,
celecoxib, combined with anticancer therapy in advanced non-small-cell lung
cancer (NSCLC) remains unclear. A meta-analysis was performed to address the
efficacy and safety of celecoxib in patients with advanced NSCLC.
Materials and
methods: Three databases, including PubMed,
EMBASE, and the Cochrane Library, were systematically searched for available
literature until March 1, 2018. Data on tumor response rates, one-year
survival, overall survival, progression-free survival, and toxicities were
extracted from the included randomized clinical trials. Subgroup analysis was carried
out according to the line of treatment. Review Manager 5.3 software was applied
to conduct the meta-analysis.
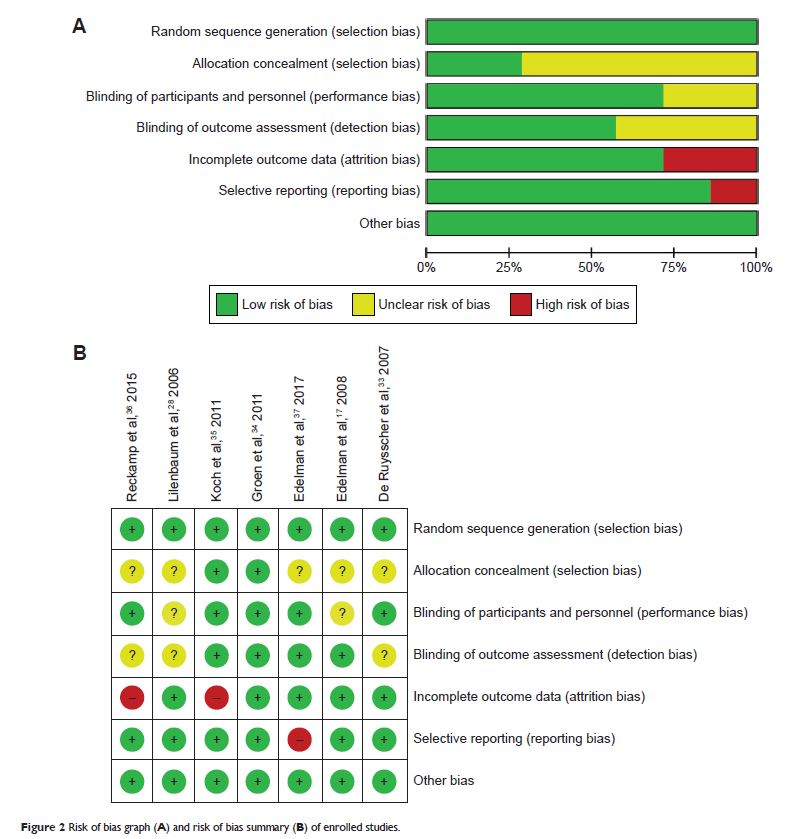
Results: A total of 7 randomized controlled trials involving 1,559 patients
with advanced NSCLC were enrolled for analysis. The pooled overall response
rate (ORR) of celecoxib added to systemic therapy was not significantly
improved (risk ratio [RR] =1.14, 95% CI =0.96–1.35, P =0.13). Additionally, no
differences were observed between the celecoxib and placebo groups regarding
1-year survival (RR =0.99, 95% CI =0.88–1.12, P =0.91).
Subgroup analysis showed that adding celecoxib to the first-line treatment
significantly improved the ORR (RR =1.21, 95% CI =1.01–1.44, P =0.04) and partial response rate
(RR =1.26, 95% CI =1.01–1.58, P =0.04). The aggregated
Kaplan–Meier analysis found no significant difference between celecoxib and
placebo regarding the 5-year overall survival (median, 12.9 vs 12.5
months, P =0.553) and 5-year
progression-free survival (median, 7.4 vs 7.2 months, P =0.641). The increased RR of
leukopenia (RR =1.25, 95% CI =1.03–1.50) and thrombocytopenia (RR =1.39, 95% CI
=1.11–1.75) indicated that celecoxib increased hematologic toxicities (grade
≥III). However, celecoxib did not increase the related risks of thrombosis or
embolism (RR =1.26, 95% CI =0.66–2.39) and cardiac ischemia (RR =1.16, 95% CI
=0.39–3.44).
Conclusion: Celecoxib had no benefit on survival indices for advanced NSCLC
but improved the ORR of first-line treatment. Additionally, celecoxib increased
the rate of hematologic toxicities without increasing the risk of
cardiovascular events.
Keywords: celecoxib, non-small-cell lung cancer, NSCLC, systematic review,
meta-analysis