108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

虫草素通过线粒体介导的内源途径诱导人胰腺癌细胞凋亡,抑制体内肿瘤生长
Authors Zhang Y, Zhang XX, Yuan RY, Ren T, Shao ZY, Wang HF, Cai WL, Chen LT, Wang XA, Wang P
Received 5 February 2018
Accepted for publication 30 May 2018
Published 1 August 2018 Volume 2018:11 Pages 4479—4490
DOI https://doi.org/10.2147/OTT.S164670
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Background: Cordycepin, the main active ingredient of a traditional Chinese
herbal remedy – extracted from Cordyceps sinensis –
has been demonstrated as a very effective anti-inflammatory and antitumor drug.
The present study investigated its antitumor effect on pancreatic cancer, a
highly aggressive cancer with extremely poor prognosis due to malignancy, and
clarified its underlying mechanism both in vitro and in vivo.
Methods: The antitumor viability of cordycepin on human pancreatic cancer
MIAPaCa-2 and Capan-1 cells was determined by colony formation assays. Annexin
V/PI double staining and flow cytometry assay were used to investigate whether
cordycepin induced apoptosis and cell cycle arrest. The mitochondrial membrane
potential (ΔΨm) was analyzed by Rhodamine 123 staining, and expression of
related proteins evaluated by Western blot and immunohistochemistry, both on
pancreatic cancer cells and tumor xenografts to reveal the potential mechanism
for the effect of cordycepin. Furthermore, the in vivo efficacy was examined on
nude mice bearing MIAPaCa-2 cell tumors treated by intraperitoneal injection of
cordycepin (0, 15, and 50 mg/kg/d) for 28 days.
Results: Cordycepin inhibited cell viability, proliferation and colony
formation ability and induced cell cycle arrest and early apoptosis of human
pancreatic cancer cells (MIAPaCa-2 and Capan-1) in a dose- and time-dependent
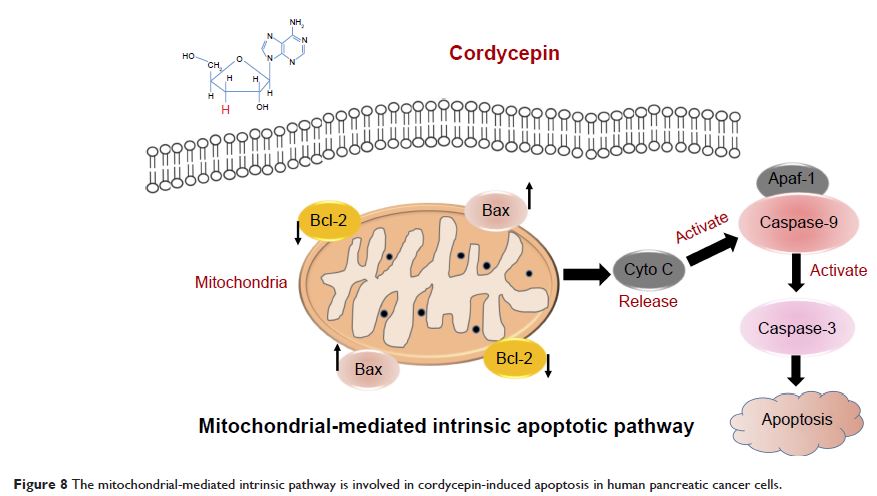
manner. The same effect was also observed in vivo. Decrease of ΔΨm and
upregulation of Bax, cleaved caspase-3, cleaved caspase-9, and cleaved PARP as
well as downregulation of Bcl-2 both in vitro and in vivo indicated that the
mitochondria-mediated intrinsic pathway was involved in cordycepin’s antitumor
effect.
Conclusion: Our data showed that cordycepin inhibited the activity of
pancreatic cancer both in vitro and in vivo by regulating apoptosis-related
protein expression through the mitochondrial pathway and suggest that
cordycepin may be a promising therapeutic option for pancreatic cancer.
Keywords: cordycepin, pancreatic cancer, proliferation, apoptosis,
mitochondrial pathway