108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

HMGB1/RAGE 轴介导肾细胞癌的细胞凋亡、侵袭、自噬和血管生成
Authors Wu CZ, Zheng JJ, Bai YH, Xia P, Zhang HC, Guo Y
Received 4 March 2018
Accepted for publication 26 April 2018
Published 1 August 2018 Volume 2018:11 Pages 4501—4510
DOI https://doi.org/10.2147/OTT.S167197
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr William Cho
Background: High mobility group box 1 protein (HMGB1) is a sort of non-histone
protein in chromatin, which plays an important role in tumor proliferation,
invasion, and immune escape. HMGB1-RAGE (receptor for advanced glycation end
products) interactions have been reported to be important in a number of
cancers.
Methods: CCK8, flow cytometry and qRT-PCR were used to detected cell
viability, apoptosis and gene expression, respectively.
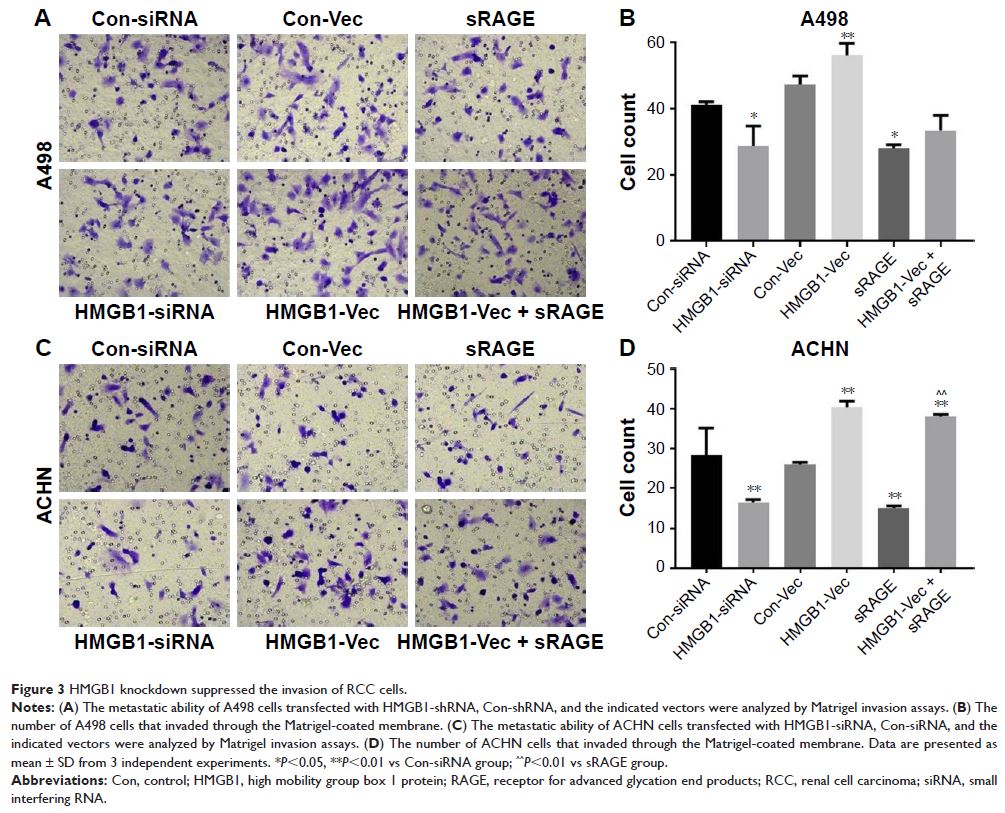
Results: In the present study, we demonstrated that HMGB1/RAGE axis
regulated the cell proliferation, apoptosis, and invasion of the renal cell
carcinoma (RCC). Further, we discovered that HMGB1/RAGE axis increased the
expression of autophagic proteins LC3 and Beclin-1 in RCC. Finally, we used a
coculture model of human umbilical vein endothelial cells with RCC cell lines
to find out that HMGB1 also increased the expression of VEGF and VEGFR2 in
human umbilical vein endothelial cells. An in vivo study further confirmed that
HMGB1 knockdown inhibited RCC tumor growth.
Conclusion: Our results illustrated that HMGB1/RAGE axis mediated RCC cell
viability, apoptosis, invasion, autophagy, and angiogenesis, which provides a
novel theoretical basis for using HMGB1 as the target in RCC.
Keywords: HMGB1, RCC, apoptosis, invasion, autophagy, angiogenesis