109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
在对局部晚期乳腺癌进行非蒽环类药物新辅助化疗的同时,进行每周一次的白蛋白结合紫杉醇与卡铂配合治疗的 II 期临床试验
Authors Huang L, Chen S, Yao L, Liu GY, Wu J, Shao ZM
Published Date March 2015 Volume 2015:10 Pages 1969—1975
DOI http://dx.doi.org/10.2147/IJN.S77000
Received 4 November 2014, Accepted 22 January 2015, Published 11 March 2015
Approved for publication by Dr Lei Yang
Background: Neoadjuvant chemotherapy has become standard treatment for women with locally advanced breast cancer. The aim of this study was to compare the efficacy and safety of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) versus paclitaxel combined with carboplatin.
Methods: Thirty patients were treated with neoadjuvant nab-paclitaxel (125 mg/m2, days 1, 8, and 15) and carboplatin (area under the curve =2; days 1, 8, and 15) every 21 days for four cycles. Ninety matched patients received paclitaxel (80 mg/m2, days 1, 8, and 15) and carboplatin every 21 days for four cycles. Weekly trastuzumab is recommended for overexpression of human epidermal receptor-2. The primary endpoint was pathologic complete response (defined as ypT0/is ypN0). Matching was conducted according to six variables: body mass index, clinical tumor stage, clinical lymph node status, estrogen receptor status, HER2 status, and trastuzumab receiving rate.
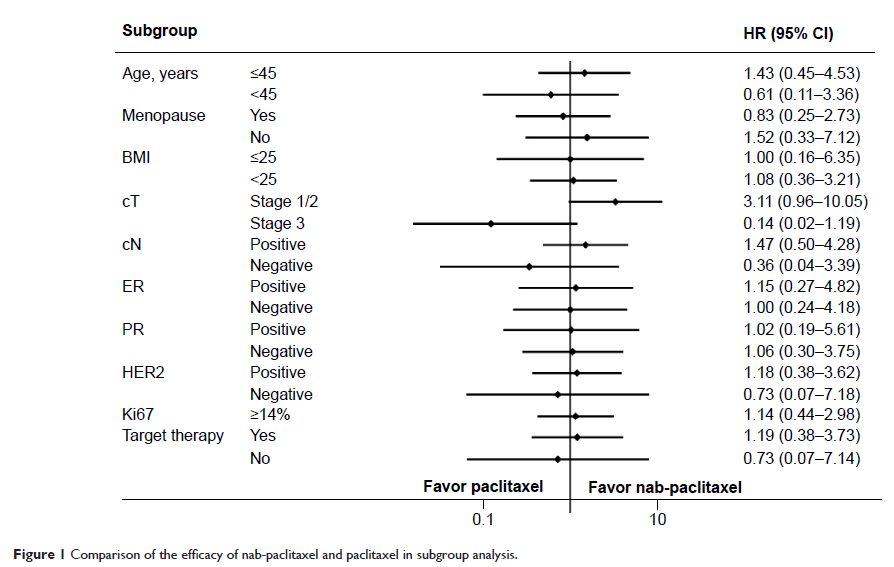
Results: Ninety percent of patients in the nab-paclitaxel group and 80% of patients in the paclitaxel group experienced a clinical objective response (complete response or partial response; P =0.450). Eight patients in the nab-paclitaxel group and 23 patients in the paclitaxel group had a pathologic complete response in the breast and axillary nodes (26.7% versus 25.6%; P =0.904). Nab-paclitaxel showed a beneficial effective trend on clinical tumor stage II (36.8% versus 15.8%; P =0.051). When trastuzumab was added to nab-paclitaxel, the pathologic complete response rate was not significantly improved more than with trastuzumab and paclitaxel (43.6% versus 39.6%; P =0.769). Carboplatin plus nab-paclitaxel or paclitaxel had similarly low pathologic complete response rates (7.7% versus 10.5%) for the luminal molecular subtype. One (50%) triple-negative patient achieved a pathologic complete response. The nab-paclitaxel regimen caused more grade 4 neutropenia than the paclitaxel regimen (56.7% versus 21.1%; P <0.001).
Conclusion: Our study shows that weekly nab-paclitaxel and carboplatin with or without trastuzumab resulted in a pathologic complete response rate that was not superior to the matched cohorts. Future, larger trials are needed to validate that nab-paclitaxel is beneficial for clinical tumor stage II and the triple-negative subgroup.
Keywords: carboplatin, nanoparticle albumin-bound paclitaxel, neoadjuvant chemotherapy, pathologic complete response
Methods: Thirty patients were treated with neoadjuvant nab-paclitaxel (125 mg/m2, days 1, 8, and 15) and carboplatin (area under the curve =2; days 1, 8, and 15) every 21 days for four cycles. Ninety matched patients received paclitaxel (80 mg/m2, days 1, 8, and 15) and carboplatin every 21 days for four cycles. Weekly trastuzumab is recommended for overexpression of human epidermal receptor-2. The primary endpoint was pathologic complete response (defined as ypT0/is ypN0). Matching was conducted according to six variables: body mass index, clinical tumor stage, clinical lymph node status, estrogen receptor status, HER2 status, and trastuzumab receiving rate.
Results: Ninety percent of patients in the nab-paclitaxel group and 80% of patients in the paclitaxel group experienced a clinical objective response (complete response or partial response; P =0.450). Eight patients in the nab-paclitaxel group and 23 patients in the paclitaxel group had a pathologic complete response in the breast and axillary nodes (26.7% versus 25.6%; P =0.904). Nab-paclitaxel showed a beneficial effective trend on clinical tumor stage II (36.8% versus 15.8%; P =0.051). When trastuzumab was added to nab-paclitaxel, the pathologic complete response rate was not significantly improved more than with trastuzumab and paclitaxel (43.6% versus 39.6%; P =0.769). Carboplatin plus nab-paclitaxel or paclitaxel had similarly low pathologic complete response rates (7.7% versus 10.5%) for the luminal molecular subtype. One (50%) triple-negative patient achieved a pathologic complete response. The nab-paclitaxel regimen caused more grade 4 neutropenia than the paclitaxel regimen (56.7% versus 21.1%; P <0.001).
Conclusion: Our study shows that weekly nab-paclitaxel and carboplatin with or without trastuzumab resulted in a pathologic complete response rate that was not superior to the matched cohorts. Future, larger trials are needed to validate that nab-paclitaxel is beneficial for clinical tumor stage II and the triple-negative subgroup.
Keywords: carboplatin, nanoparticle albumin-bound paclitaxel, neoadjuvant chemotherapy, pathologic complete response