108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

对糖尿病患者应用吡格列酮与膀胱癌风险:系统综述和荟萃分析
Authors Yan H, Xie H, Ying Y, Li J, Wang X, Xu X, Zheng X
Received 7 February 2018
Accepted for publication 11 April 2018
Published 22 June 2018 Volume 2018:10 Pages 1627—1638
DOI https://doi.org/10.2147/CMAR.S164840
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Justinn Cochran
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Abstract: Pioglitazone
has been reported to increase the risk of bladder cancer but the conclusions of
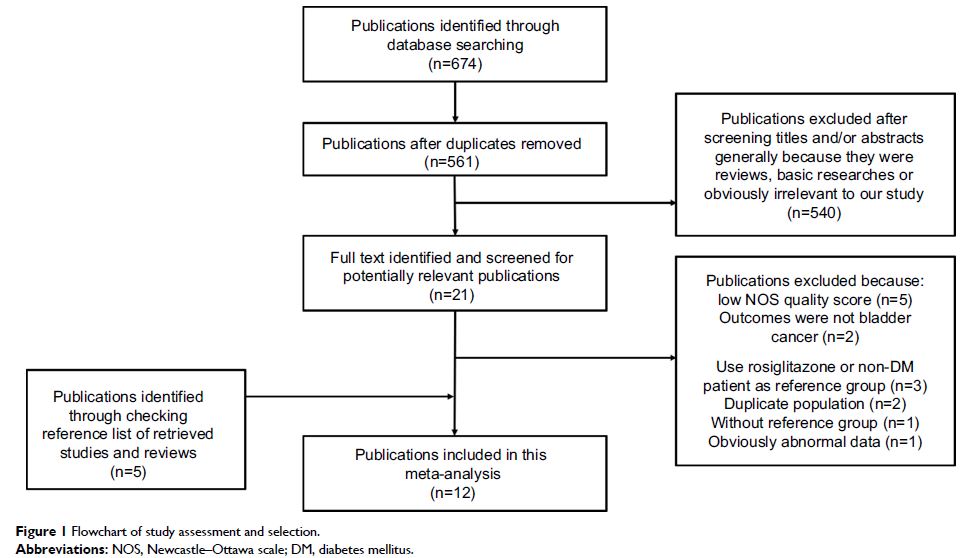
published clinical studies are confusing. We conducted a systematic review and
meta-analysis of all eligible randomized controlled trial (RCT) studies and
observational studies, in order to identify a more precise relationship between
pioglitazone and risk of bladder cancer. We searched for publications up to
January 24, 2018, in PubMed, EMBASE, Scopus, Web of Science, Cochrane register,
and Chinese National Knowledge Infrastructure databases, and the references of
the retrieved articles and relevant reviews were also checked. Relative risk
and 95% confidence interval (CI) were used to assess this correlation. A
dose-related meta-analysis was performed as well. Data on RCT studies showed a
null association between pioglitazone and bladder cancer. The pooled RR
estimates of the 12 included studies illustrated that pioglitazone is associated
with a 14% increased risk of bladder cancer (95% CI 1.03–1.26). No evidence of
publication bias was detected. In the dose effect analysis, patients who used a
higher dose of pioglitazone had an increased risk of bladder cancer. In
conclusion, this meta-analysis indicated that pioglitazone is associated with
an increased risk of bladder cancer. Further research should be conducted to
confirm our findings and reveal the potential biological mechanisms.
Keywords: thiazolidinedione, bladder tumor, epidemiology, dose effect, risk
factor