108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

八聚精氨酸(Octaarginine)修饰的金纳米粒子增强人结直肠癌细胞系 LS180 对兆伏辐射的放射敏感性
Authors Zhang XY, Wang H, Coulter JA, Yang R
Received 30 December 2017
Accepted for publication 26 April 2018
Published 19 June 2018 Volume 2018:13 Pages 3541—3552
DOI https://doi.org/10.2147/IJN.S161157
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Mohankandhasamy Ramasamy
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Background: This study investigated the effectiveness and underpinning
mechanisms of radiosensitization using octaarginine (R8)-modified gold
nanoparticle–poly(ethylene glycol) (GNP-PEG-R8) in colorectal cancer cell line
LS180 to megavoltage radiotherapy in vitro.
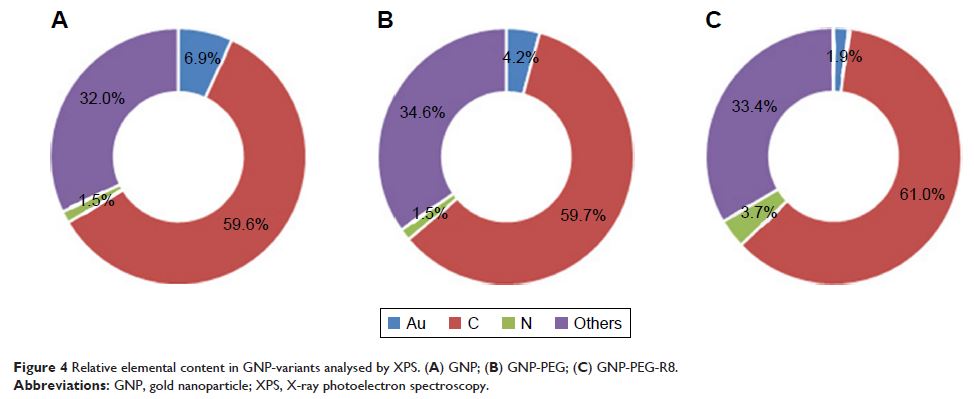
Method: In-house synthesized GNP-PEG was characterized
by transmission electron microscopy, dynamic light scattering,
ultraviolet–visible spectrophotometry, and X-ray photoelectron spectroscopy.
Inductively coupled plasma mass spectroscopy was used to quantify
internalization. Direct cytotoxicity was established using the Cell Counting
Kit-8, while radiosensitivity was determined using the gold standard in vitro
clonogenic assay. Cell-cycle distribution, apoptosis, reactive oxygen species
(ROS), and mitochondrial membrane potential (MMP) were analyzed by flow
cytometry, further exploring the key mechanisms driving GNP-PEG-R8
radiosensitization.
Results: The core GNP diameter was 6.3±1.1 nm
(mean±SD). Following functionalization, the hydrodynamic diameter increased to
19.7±2.8 nm and 27.8±1.8 nm for GNP-PEG and GNP-PEG-R8, with respective
surface plasmon resonance peaks of 515 nm and 525 nm. Furthermore,
incorporation of the R8 significantly increased nanoparticle internalization
compared to GNP-PEG (p <0.001) over a
1 h treatment period. Functionalized GNPs confer little cytotoxicity below
400 nM. In clonogenic assays, radiation combined with GNP-PEG-R8 induced a
significant reduction in colony formation compared with radiation alone,
generating a sensitizer enhancement ratio of 1.59. Furthermore, GNP-PEG-R8 plus
radiation predominantly induced cell-cycle arrest in the G2/M phase, increasing
G2/M stalling by an additional 10% over GNP-PEG, markedly promoting apoptosis (p <0.001). Finally, ROS levels
and alterations in MMP were investigated, indicating a highly significant (p <0.001) change in both
parameters following the combined treatment of GNP-PEG-R8 and radiation over
radiation alone.
Conclusion: R8-modified GNPs were efficiently internalized
by LS180 cells, exhibiting minimal cytotoxicity. This yielded significant
radiosensitization in response to megavoltage radiation. GNP-PEG-R8 may enhance
radiosensitivity by arresting cell cycle and inducing apoptosis, with elevated
ROS identified as the likely initiator.
Keywords: gold
nanoparticles, octaarginine, colorectal cancer, megavoltage radiotherapy,
mechanisms, radiosensitization