108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

洛莫司汀和贝伐单抗对进行性胶质母细胞瘤的疗效:一项综合分析
Authors Song J, Xue YQ, Zhao MM, Xu P
Received 23 December 2017
Accepted for publication 1 March 2018
Published 13 June 2018 Volume 2018:11 Pages 3435—3439
DOI https://doi.org/10.2147/OTT.S160685
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Background: Glioblastomas (GBMs) are the most aggressive type of glial brain tumors.
Despite aggressive treatment with surgery and chemoradiation, GBMs invariably
relapse and tumors are progressive. Controversy remains on optimal treatment of
patients with recurrent GBMs. Data from previous trials have suggested that the
addition of bevacizumab (BEV) to lomustine (CCNU) might improve overall
survival (OS) as compared with that with monotherapies. The aim of this study
was to compare the efficacy of BEV in addition to CCNU versus single-agent
therapy in patients with recurrent GBM.
Methods: Electronic databases were searched for eligible literature updated
in December 2017. Trials assessing the effectiveness of CCNU and BEV in
progressive GBM were included, of which the main outcomes were progression-free
survival (PFS) and OS. All the data were pooled with the corresponding 95%
confidence intervals (CIs) using RevMan software. Sensitivity and heterogeneity
were quantitatively evaluated.
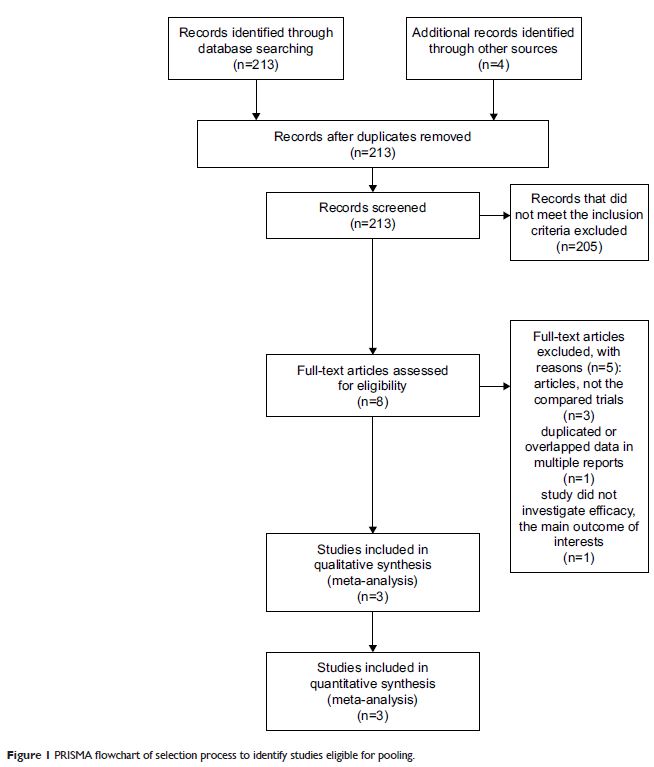
Results: Three randomized clinical trials were identified, including 574
patients (combination group: 358, monotherapies group: 216). The combination
group treated with BEV and CCNU showed improvement in PFS (OR = 0.49; 95% CI,
0.41–0.59; p < 0.00001). No
significant differences were, however, found in patients in terms of the OS (OR
= 0.84; 95% CI, 0.68–1.03; p = 0.09).
Conclusion: Although treatment with CCNU plus BEV prolonged PFS, it did not
confer OS advantage over monotherapies in patients with progressive GBM. The
encouraging results of the addition of CCNU to BEV warrant investigation in
further randomized trials.
Keywords: glioblastoma, bevacizumab, lomustine, meta-analysis, brain tumors,
progression free survival