108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Inhaled glycopyrrolate for the treatment of chronic obstructive pulmonary disease
Authors Tashkin DP, Gross NJ
Received 16 January 2018
Accepted for publication 19 April 2018
Published 12 June 2018 Volume 2018:13 Pages 1873—1888
DOI https://doi.org/10.2147/COPD.S162646
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Charles Downs
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
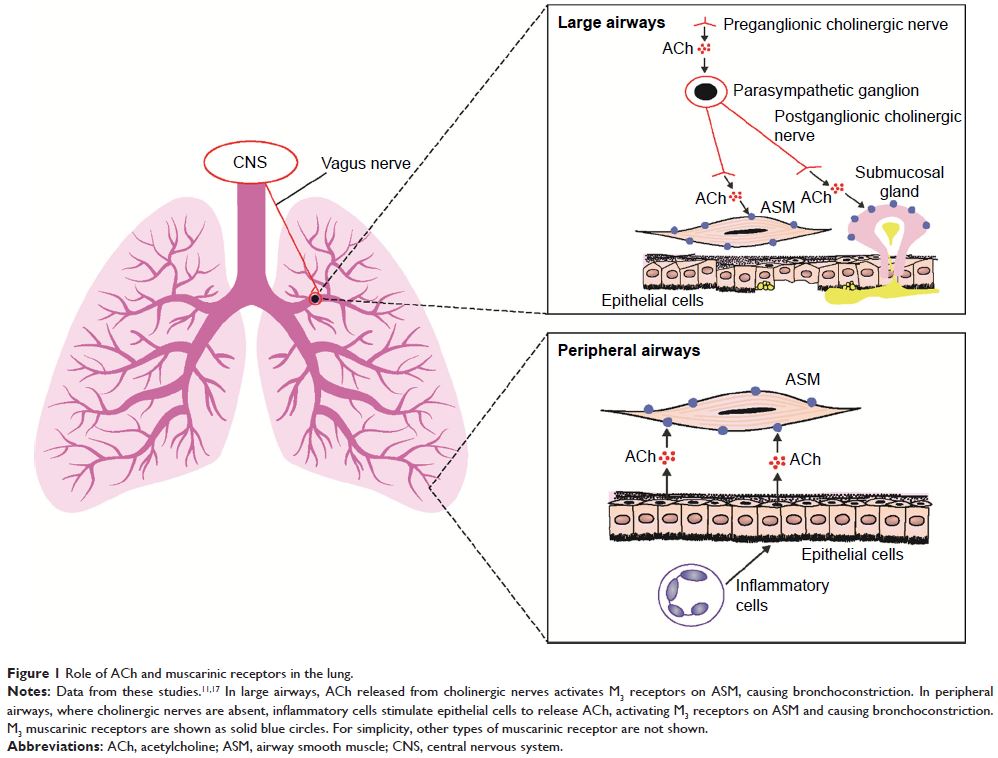
Abstract: Long-acting muscarinic
antagonists (LAMAs), along with long-acting β2-agonists
(LABAs), are the mainstay for treatment of patients with COPD. Glycopyrrolate,
or glycopyrronium bromide, like other LAMAs, inhibits parasympathetic nerve
impulses by selectively blocking the binding of acetylcholine to muscarinic
receptors. Glycopyrrolate is unusual in that it preferentially binds to M3 over M2 muscarinic
receptors, thereby specifically targeting the primary muscarinic receptor
responsible for bronchoconstriction occurring in COPD. Inhaled glycopyrrolate
is slowly absorbed from the lungs and rapidly eliminated from the bloodstream,
most likely by renal excretion in its unmetabolized form, limiting the
potential for systemic adverse events. Inhaled glycopyrrolate is a fast-acting,
efficacious treatment option for patients with moderate–severe COPD. It
improves lung function, reduces the risk of exacerbations, and alleviates the
symptoms of breathlessness, which in turn may explain the improvement seen in
patients’ quality of life. Inhaled formulations containing glycopyrrolate are
well tolerated, and despite being an anticholinergic, few cardiovascular-related
events have been reported. Inhaled glycopyrrolate is thus of value as both
monotherapy and in combination with other classes of medication for maintenance
treatment of COPD. This review covers the mechanism of action of inhaled glycopyrrolate,
including its pharmacokinetic, pharmacodynamic, and safety profiles, and
effects on mucus secretion. It also discusses the use of inhaled glycopyrrolate
in the treatment of COPD, as monotherapy and in fixed-dose combinations with
LABAs and inhaled corticosteroid–LABAs, including a triple therapy recently
approved in Europe.
Keywords: glycopyrronium
bromide, long-acting muscarinicantagonist, anticholinergic, bronchodilator