108605
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
在癌症患者中使用程序性细胞死亡受体-1 抑制剂的血液学毒性风险:对当前研究的综合分析
Authors Sui JD, Wang Y, Wan Y, Wu YZ
Received 2 March 2018
Accepted for publication 18 April 2018
Published 8 June 2018 Volume 2018:12 Pages 1645—1657
DOI https://doi.org/10.2147/DDDT.S167077
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Background: Programmed cell
death-1 (PD-1) inhibitor-related hematologic toxicities are a category of rare
but clinically serious and potentially life-threatening adverse events;
however, little is known about their risks across different treatment regimens
and tumor types. The objective of this study was to compare the incidences of
PD-1 inhibitor-related hematologic toxicities among different therapeutic
regimens and tumor types.
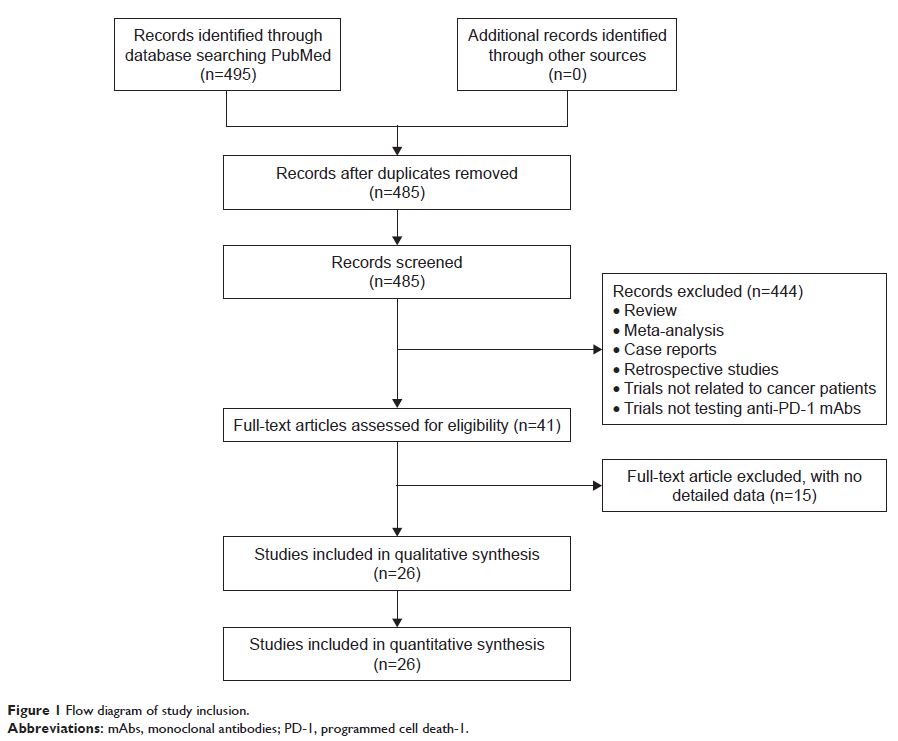
Methods: Twenty-six original articles on PD-1 inhibitor trials were identified based on a PubMed search completed on September 26, 2017. The incidences of hematologic toxicities were collected.
Results: A total of 26 studies containing 5,088 patients were included in the meta-analysis. PD-1 inhibitor monotherapy was associated with an increased risk of all-grade anemia in cancer patients (5%, 95% CI 4%–6%), particularly in patients with renal cell carcinoma (RCC) (8%, 95% CI 6%–12%), compared with all-grade thrombocytopenia (2%, 95% CI 1%–5%), leukopenia (2%, 95% CI 1%–3%), and neutropenia (1%, 95% CI 0–1%). However, low incidences of high-grade hematologic toxicities were observed in cancer patients treated with PD-1 inhibitor monotherapy. The use of PD-1 inhibitors in combination with ipilimumab, peptide vaccines, or chemotherapy had significantly higher risks than PD-1 inhibitor monotherapy for all-grade anemia (13%, 95% CI 5%–31%), thrombocytopenia (6%, 95% CI 2%–18%), leukopenia (5%, 95% CI 1%–35%), neutropenia (4%, 95% CI 1%–26%), and only high-grade thrombocytopenia (4%, 95% CI 1%–15%). In addition, all-grade and high-grade hematologic toxicities in chemotherapy and everolimus treatment arms were more frequent than in PD-1 inhibitor monotherapy arms.
Conclusion: The risks of PD-1 inhibitor-related hematologic toxicities were higher in RCC than in other cancers, and during combination therapy. These results may contribute toward enhancing awareness among clinicians about frequent clinical monitoring when managing PD-1 inhibitors.
Keywords: nivolumab, pembrolizumab, immunotherapy, hematological adverse events
Methods: Twenty-six original articles on PD-1 inhibitor trials were identified based on a PubMed search completed on September 26, 2017. The incidences of hematologic toxicities were collected.
Results: A total of 26 studies containing 5,088 patients were included in the meta-analysis. PD-1 inhibitor monotherapy was associated with an increased risk of all-grade anemia in cancer patients (5%, 95% CI 4%–6%), particularly in patients with renal cell carcinoma (RCC) (8%, 95% CI 6%–12%), compared with all-grade thrombocytopenia (2%, 95% CI 1%–5%), leukopenia (2%, 95% CI 1%–3%), and neutropenia (1%, 95% CI 0–1%). However, low incidences of high-grade hematologic toxicities were observed in cancer patients treated with PD-1 inhibitor monotherapy. The use of PD-1 inhibitors in combination with ipilimumab, peptide vaccines, or chemotherapy had significantly higher risks than PD-1 inhibitor monotherapy for all-grade anemia (13%, 95% CI 5%–31%), thrombocytopenia (6%, 95% CI 2%–18%), leukopenia (5%, 95% CI 1%–35%), neutropenia (4%, 95% CI 1%–26%), and only high-grade thrombocytopenia (4%, 95% CI 1%–15%). In addition, all-grade and high-grade hematologic toxicities in chemotherapy and everolimus treatment arms were more frequent than in PD-1 inhibitor monotherapy arms.
Conclusion: The risks of PD-1 inhibitor-related hematologic toxicities were higher in RCC than in other cancers, and during combination therapy. These results may contribute toward enhancing awareness among clinicians about frequent clinical monitoring when managing PD-1 inhibitors.
Keywords: nivolumab, pembrolizumab, immunotherapy, hematological adverse events