108899
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

戊酸雌二醇/地诺孕素在健康女性人群中的避孕效果和安全性:一项多中心,开放标签,非对照 III 期研究
Authors Yu Q, Huang ZR, Ren ML, Chang Q, Zhang Z, Parke S
Received 14 November 2017
Accepted for publication 25 February 2018
Published 7 June 2018 Volume 2018:10 Pages 257—266
DOI https://doi.org/10.2147/IJWH.S157056
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Leyla Bahar
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Background: To investigate the efficacy and safety of a combined oral
contraceptive containing estradiol valerate and dienogest (EV/DNG) in healthy
Asian women.
Methods: In this multicenter Phase III study,
women received oral EV/DNG in a 28-day regimen for 13 cycles. The primary
efficacy endpoint was the number of unintended pregnancies, measured by the
Pearl Index (PI); secondary efficacy endpoints included bleeding pattern and
cycle control parameters. Adverse events were monitored during the study and
overall satisfaction with treatment was determined on completion of the study.
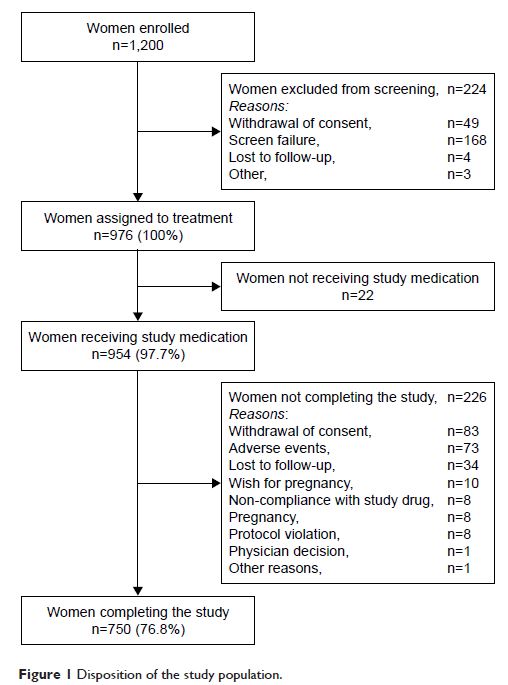
Results: A total of 954 Asian women (97.7% of subjects assigned to study
medication; mean age 33.4 years) were treated. Five pregnancies were reported
during EV/DNG treatment over 796.34 relevant woman-years of exposure, giving an
unadjusted PI of 0.63 and a cumulative failure rate of 0.0049; 3 pregnancies
during EV/DNG treatment over 760.35 relevant woman-years of exposure gave an
adjusted PI of 0.39. The bleeding pattern improved during the reporting periods
within the study. The proportion of women who experienced withdrawal bleeding
decreased with treatment (84.9% of women during Cycle 1 vs 79.3% in Cycle 13),
and the mean length of withdrawal bleeding decreased with treatment (4.2 vs 3.4
days). The number and maximum length of intracyclic bleeding/spotting episodes
also decreased with EV/DNG. EV/DNG was well tolerated, and 92% of women
included in the study were very satisfied or somewhat satisfied with EV/DNG.
Conclusion: EV/DNG showed high contraceptive efficacy, was well tolerated in Asian
women, and may be effectively used in this population.
Clinical
trials registry: ClinicalTrial.gov identifier:
NCT01638910.
Keywords: Asian, bleeding pattern, combined oral contraceptive, cycle control,
estradiol valerate/dienogest, women