109669
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
作为多靶点抗老年痴呆症候选药物的脑苷肌肽和肌肽注射液可以改善 APPSWE/ PS1dE9 小鼠的认知功能障碍
Authors Gao Y, Hu YZ, Li RS, Han ZT, Geng Y, Xia Z, Du WJ, Liu LX, Zhang HH, Wang LN
Published Date February 2015 Volume 2015:11 Pages 537—548
DOI http://dx.doi.org/10.2147/NDT.S78025
Received 24 November 2014, Accepted 21 January 2015, Published 27 February 2015
Background: Cattle encephalon glycoside and ignotin injection (CEGI), a
multitargeted neurotrophic drug, has been widely used in the treatment of
central and peripheral nerve injuries, such as stroke, hypoxic ischemic
encephalopathy, and diabetic neuropathy in the People’s Republic of China.
However, data regarding the effect of CEGI on Alzheimer’s disease (AD) remain
scarce. The present study aimed to investigate the effect of CEGI on learning
and memory in an APPswe/PS1dE9 double-transgenic mouse model, a suitable animal
model of AD, and elucidate its possible mechanisms.
Materials and methods: Five-month-old APP/PS1 mice were intraperitoneally administered 6.6 mL/kg or 13.2 mL/kg of CEGI for 1 month. After 1 month of administration, all mice received Morris water maze training and a probe test. Mouse brain sections were detected by standard biochemical and immunohistochemical measures.
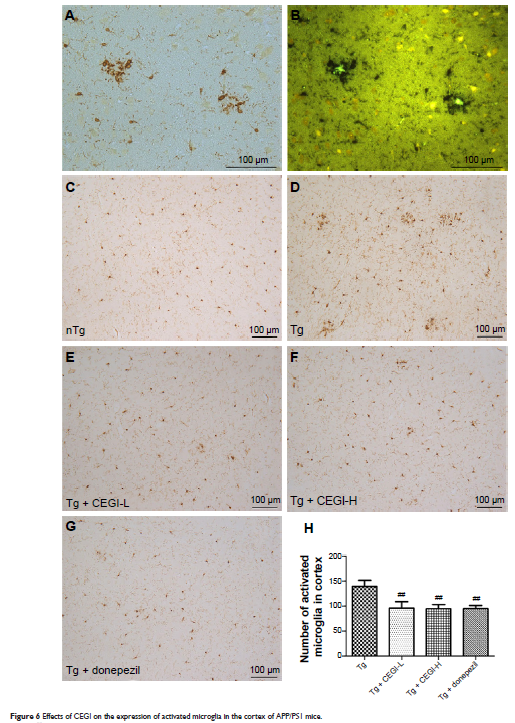
Results: CEGI treatment significantly improved the spatial learning and memory deficits and decreased cerebral amyloid-β42 levels in brain homogenates of APP/PS1 mice. CEGI treatment elevated the activities of superoxide dismutase, and reduced the levels of malondialdehyde. CEGI attenuated neuronal damage in the hippocampus of APP/PS1 mice and upregulated protein and gene expression of Bcl-2 and the ratio of Bcl-2/Bax. CEGI treatment decreased the number of Iba1+ activated microglia in the cortex of the APP/PS1 mice.
Conclusion: Our results showed that CEGI prevents memory impairment, possibly by decreasing the amyloid-β42 levels in APP/PS1 mice and inhibiting oxidative stress, apoptosis, and inflammation, making CEGI a promising therapeutic agent for AD.
Keywords: Alzheimer’s disease, cognitive impairment, amyloid-β, oxidative stress, apoptosis, inflammation
Materials and methods: Five-month-old APP/PS1 mice were intraperitoneally administered 6.6 mL/kg or 13.2 mL/kg of CEGI for 1 month. After 1 month of administration, all mice received Morris water maze training and a probe test. Mouse brain sections were detected by standard biochemical and immunohistochemical measures.
Results: CEGI treatment significantly improved the spatial learning and memory deficits and decreased cerebral amyloid-β42 levels in brain homogenates of APP/PS1 mice. CEGI treatment elevated the activities of superoxide dismutase, and reduced the levels of malondialdehyde. CEGI attenuated neuronal damage in the hippocampus of APP/PS1 mice and upregulated protein and gene expression of Bcl-2 and the ratio of Bcl-2/Bax. CEGI treatment decreased the number of Iba1+ activated microglia in the cortex of the APP/PS1 mice.
Conclusion: Our results showed that CEGI prevents memory impairment, possibly by decreasing the amyloid-β42 levels in APP/PS1 mice and inhibiting oxidative stress, apoptosis, and inflammation, making CEGI a promising therapeutic agent for AD.
Keywords: Alzheimer’s disease, cognitive impairment, amyloid-β, oxidative stress, apoptosis, inflammation