108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

智能聚合物纳米粒子具有 pH 响应性和 PEG 可分离特性,可共同输送紫杉醇和生存素 siRNA 以增强抗肿瘤效果
Authors Jin M, Jin G, Kang L, Chen L, Gao Z, Huang W
Received 3 January 2018
Accepted for publication 17 February 2018
Published 20 April 2018 Volume 2018:13 Pages 2405—2426
DOI https://doi.org/10.2147/IJN.S161426
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: The co-delivery of chemotherapeutic agents and small interfering RNA
(siRNA) within one cargo can enhance the anticancer outcomes through its
synergistic therapeutic effects.
Materials and
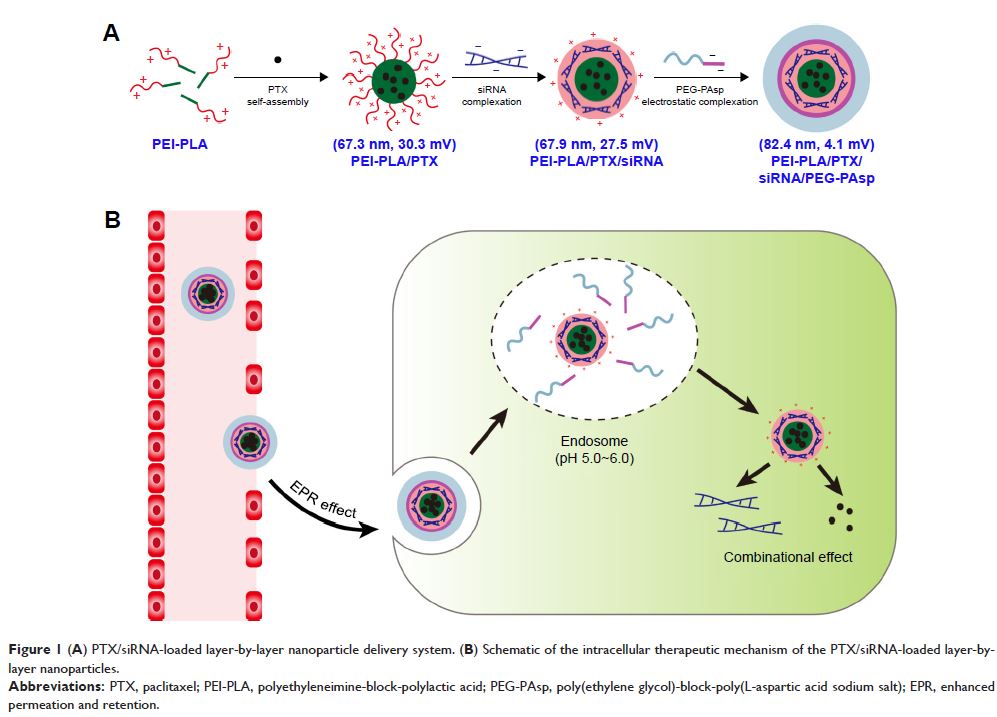
methods: We prepared smart polymeric
nanoparticles (NPs) with pH-responsive and poly(ethylene glycol)
(PEG)-detachable properties to systemically co-deliver paclitaxel (PTX) and
siRNA against survivin gene for lung cancer therapy. The cationic
polyethyleneimine-block-polylactic acid (PEI-PLA) was first synthesized and
characterized, with good biocompatibility. PTX was encapsulated into the
hydrophobic core of the PEI-PLA polymers by dialysis, and then the survivin
siRNA was loaded onto the PTX-loaded NPs (PEI-PLA/PTX) through electrostatic
interaction between siRNA and PEI block. Finally, the negatively charged
poly(ethylene glycol)-block-poly(l-aspartic acid sodium salt) (PEG-PAsp) was
coated onto the surface of NPs by electrostatic interaction to form final smart
polymeric NPs with mean particle size of 82.4 nm and zeta potential of 4.1 mV.
After uptake of NPs by tumor cells, the PEG-PAsp segments became electrically
neutral owing to the lower endosome pH and consequently detached from the smart
NPs. This process allowed endosomal escape of the NPs through the proton-sponge
effect of the exposed PEI moiety.
Results: The resulting NPs achieved drug loading of 6.04 wt% and exhibited
good dispersibility within 24 h in 10% fetal bovine serum (FBS). At pH
5.5, the NPs presented better drug release and cellular uptake than at pH 7.4.
The NPs with survivin siRNA effectively knocked down the expression of survivin
mRNA and protein owing to enhanced cell uptake of NPs. Cell counting kit-8
(CCK-8) assay showed that the NPs presented low systemic toxicity and improved
antiproliferation effect of PTX on A549 cells. Moreover, in vivo studies
demonstrated that accumulated NPs in the tumor site were capable of inhibiting
the tumor growth and extending the survival rate of the mice by silencing the
survivin gene and delivering PTX into tumor cells simultaneously.
Conclusion: These results indicate that the prepared nano-vectors could be a
promising co-delivery system for novel chemo/gene combination therapy.
Keywords: PEG detachable, co-delivery, survivin siRNA, paclitaxel, pH
responsive