108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

双重抗血小板治疗轻微卒中或 TIA 复发性卒中的效果取决于体重
Authors Ma Y, Liu Y, Xu J, Wang YL, Wang YJ, Du FH
Received 10 November 2017
Accepted for publication 29 December 2017
Published 8 May 2018 Volume 2018:14 Pages 861—870
DOI https://doi.org/10.2147/TCRM.S156694
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Hoa Le
Peer reviewer comments 2
Editor who approved publication: Professor Deyun Wang
Objective: To
assess whether bodyweight influences the efficacy and safety of dual
antiplatelet therapy (DAT) in male patients with minor stroke or transient
ischemic attack patients.
Materials and
methods: All 3,420 male participants coming
from the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular
Events trial were divided into 3 groups based on bodyweight (<65 kg, 65–75
kg, and ≥75 kg). The stroke outcomes included stroke recurrence, combined
vascular events, and bleeding events during 90 days of follow-up. The
interaction of the treatment effects of DAT among patients with different
bodyweight was assessed by Cox proportional hazards models.
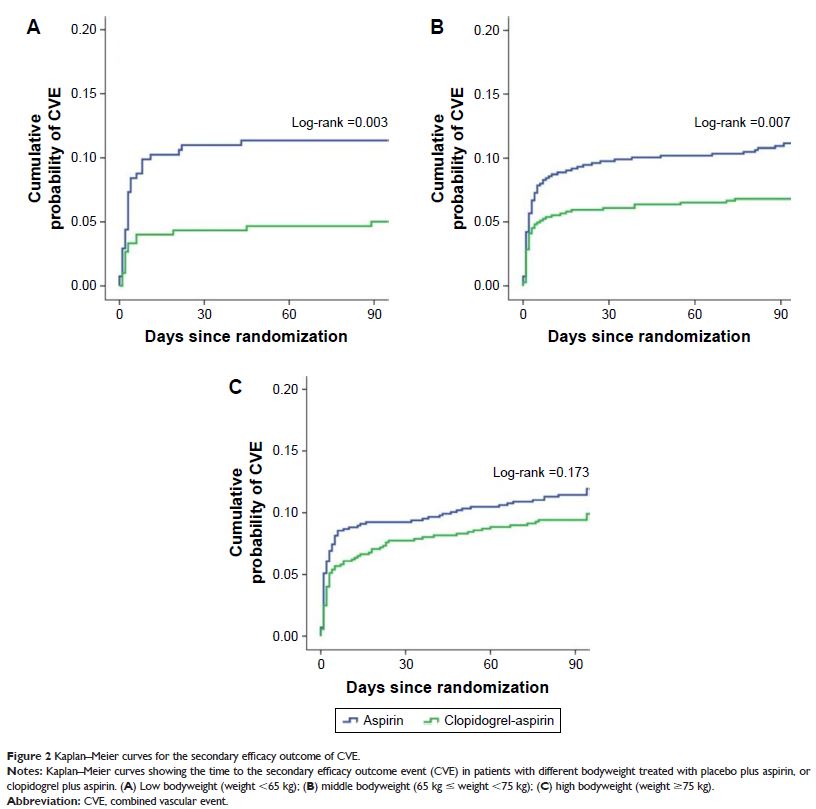
Results: DAT is superior to mono antiplatelet therapy (MAT) for reducing
stroke recurrence among patients with weight <65 kg (5.0% vs 11.7%; hazard
ratio [HR], 0.41; 95% CI: 0.22–0.76) and 65–75 kg (6.7% vs 10.8%, HR, 0.62; 95%
CI: 0.43–0.89). However, no significant difference was found in stroke
recurrence between DAT and MAT in patients with weight ≥75 kg (9.4% vs
11.6%; HR, 0.80; 95% CI: 0.58–1.10). A significant interaction was observed
between weight and antiplatelet therapy on stroke recurrence (p <0.05). Similar result was
found for combined vascular events. More bleeding events were found in DAT
group among patients with weight <65 kg (3.7% vs 2.2%), but with no
significant difference.
Conclusion: DAT does not show benefit in patients with higher weight, compared
with MAT. Bleeding events found in the DAT group were not more than the MAT
group among patients with lower weight.
Clinical trial
registration: URL: http://www.ClinicalTrials.gov. Unique identifier: NCT00979589.
Keywords: bodyweight, dual antiplatelet therapy, ischemic stroke, outcomes,
TIA