108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

miR-488 通过靶向 HMGN5 来抑制肾细胞癌中的细胞生长和转移
Authors Wei X, Yu L, Kong X
Received 7 November 2017
Accepted for publication 27 February 2018
Published 18 April 2018 Volume 2018:11 Pages 2205—2216
DOI https://doi.org/10.2147/OTT.S156361
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Purpose: microRNAs are thought to play crucial roles in tumorigenesis.
Dysregulation of miR-488 has been implicated to be involved in several cancer
progressions. However, the biological functions of miR-488 in renal cell
carcinoma (RCC) remain unclear. This study aimed to explore the molecular
mechanism underlying the role of miR-488 in RCC development.
Materials and
methods: The expression levels of miR-488
were detected in 38 paired RCC tumor samples and cell lines by quantitative
real-time polymerase chain reaction method. miR-488 was upregulated by mimics
transfection in RCC cell lines. MTT, colony formation, transwell assay, flow
cytometry assay, and a xenograft model were performed to determine cell
proliferation, invasion, migration, epithelial-to-mesenchymal transition, and
apoptosis in vitro and in vivo. Moreover, the potential target of miR-488 was
verified by dual-luciferase reporter assay, quantitative real-time polymerase
chain reaction, and Western blot. The correlation between miR-488 expression
and its target gene expression was confirmed by Spearman’s correlation analysis
in 38 selected RCC tissue samples.
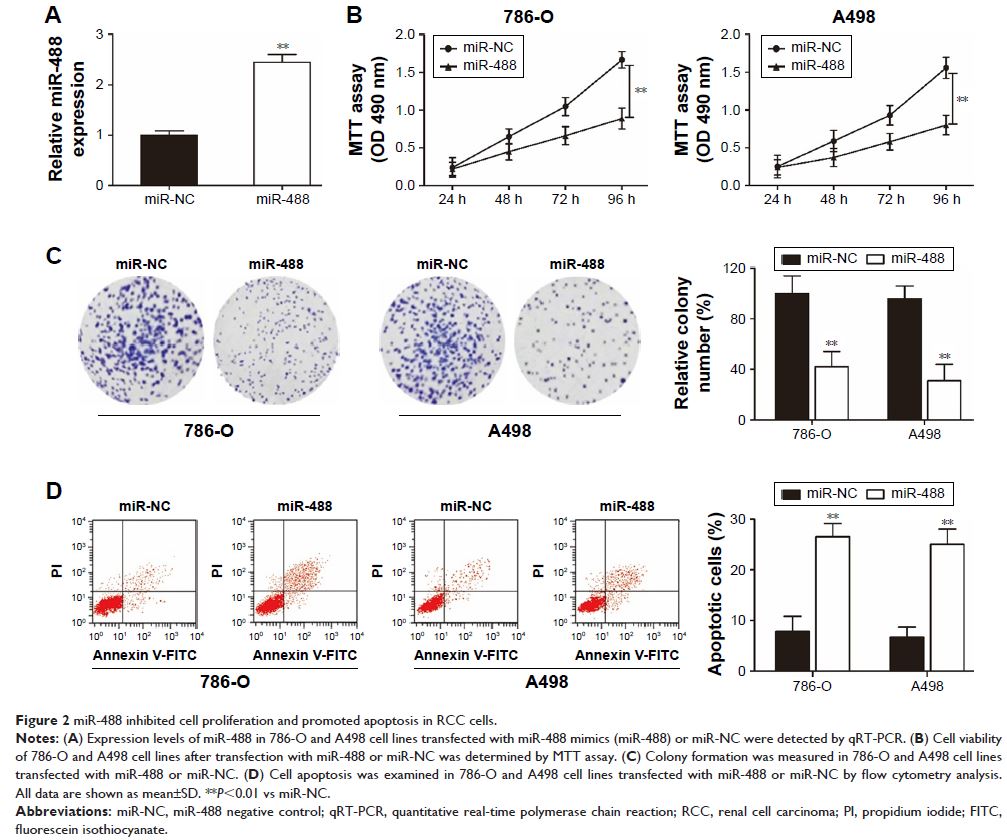
Results: We found that miR-488 was remarkably downregulated in human RCC
samples and cell lines compared with paired normal tissues and cell lines.
Functional investigations revealed that overexpression of miR-488 significantly
suppressed cell proliferation, invasion, and migration, and promoted cell
apoptosis in RCC cells. Nucleosome binding protein 1 (high-mobility group
nucleosome binding domain 5 [HMGN5]) was identified as a direct target of
miR-488, and an inverse relationship was found between miR-488 expression and
HMGN5 mRNA levels in RCC specimens. Rescue experiments suggested that
restoration of HMGN5 partially abolished miR-488-mediated cell proliferation
and invasion inhibition in RCC cells through regulating phosphatidylinositol
3-kinase/protein kinase B/the mammalian target of rapamycin and
epithelial-to-mesenchymal transition signaling pathways.
Conclusion: These data indicated that miR-488 acted as a tumor suppressor in
RCC proliferation and invasion by targeting HMGN5, which might provide
potential therapeutic biomarker for RCC patients.
Keywords: renal cell carcinoma, miRNA-488, HMGN5, metastasis, proliferation