108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
通过 DNA 损伤应答和自噬途径,抑制 NIPBL 可增强非小细胞肺癌细胞的化学敏感性
Authors Zheng L, Zhou H, Guo L, Xu X, Zhang S, Xu W, Mao W
Received 1 December 2017
Accepted for publication 10 February 2018
Published 5 April 2018 Volume 2018:11 Pages 1941—1948
DOI https://doi.org/10.2147/OTT.S158655
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Federico Perche
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Background: Previously, we
reported that high expression of nipped-B-like protein (NIPBL) was strongly
correlated with poor prognosis, tumor differentiation, and lymph node
metastasis. Survival analysis indicated that NIPBL expression was a potential
prognostic factor for non-small cell lung cancer (NSCLC). Moreover, loss of
NIPBL decreased lung cancer cells proliferation, migration, invasion and
promoted apoptosis as well as sensitivity to chemotherapeutic agents. However,
the deep mechanisms were not explored.
Purpose: The objective of this study was to identify the role of NIPBL in DNA damage response, as well as autophagy pathway, so as to interpret the mechanisms of how NIPBL knockdown enhances the chemosensitivity of lung cancer cell.
Methods: Cells (NCI-H1299 and NCI-H1650) were transfected by specific siRNAs before immunofluorescence and single-cell gel electrophoresis, which were mainly used to observe the differences of DNA damage in different groups. Additionally, protein were obtained and then analyzed by western blot and mass spectroscopy.
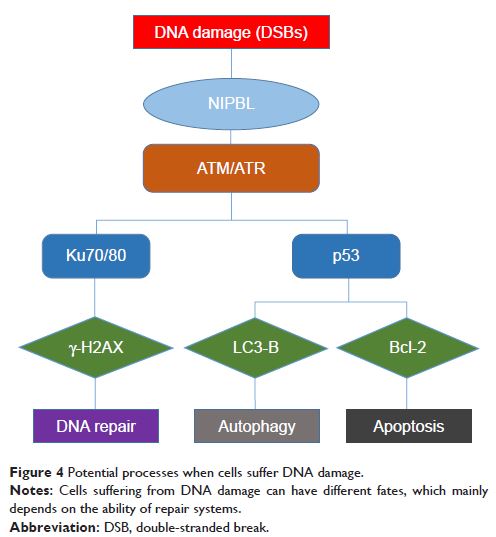
Results: In this study, we found that knockdown of NIPBL resulted in accumulation of phosphorylated H2AX (γ-H2AX) foci and higher levels of DNA damage, as revealed by comet assay. Western blot assay revealed that loss of NIPBL decreased expression of ATM/ATR, Rad3-related protein and Ku70/Ku80, but increased expression of LC3-B and depletion of p62. Using mass spectroscopy, we identified eight proteins that were significantly differentially expressed upon NIPBL knockdown. Gene Ontology analysis revealed that these proteins are mainly involved in DNA repair, mismatch repair, and binding to damaged DNA. The expression changes in two of the proteins, MSH2 and STAT1, were verified by Western blotting in NIPBL-knockdown cells.
Conclusions: In summary, these results reflected that loss of NIPBL impairs the DNA damage response and promotes autophagy. And NIPBL suppression may represent a novel strategy for preventing chemotherapy resistance in lung cancer.
Keywords: nipped-B-like protein, lung cancer, DNA damage response, double-strand break, autophagy
Purpose: The objective of this study was to identify the role of NIPBL in DNA damage response, as well as autophagy pathway, so as to interpret the mechanisms of how NIPBL knockdown enhances the chemosensitivity of lung cancer cell.
Methods: Cells (NCI-H1299 and NCI-H1650) were transfected by specific siRNAs before immunofluorescence and single-cell gel electrophoresis, which were mainly used to observe the differences of DNA damage in different groups. Additionally, protein were obtained and then analyzed by western blot and mass spectroscopy.
Results: In this study, we found that knockdown of NIPBL resulted in accumulation of phosphorylated H2AX (γ-H2AX) foci and higher levels of DNA damage, as revealed by comet assay. Western blot assay revealed that loss of NIPBL decreased expression of ATM/ATR, Rad3-related protein and Ku70/Ku80, but increased expression of LC3-B and depletion of p62. Using mass spectroscopy, we identified eight proteins that were significantly differentially expressed upon NIPBL knockdown. Gene Ontology analysis revealed that these proteins are mainly involved in DNA repair, mismatch repair, and binding to damaged DNA. The expression changes in two of the proteins, MSH2 and STAT1, were verified by Western blotting in NIPBL-knockdown cells.
Conclusions: In summary, these results reflected that loss of NIPBL impairs the DNA damage response and promotes autophagy. And NIPBL suppression may represent a novel strategy for preventing chemotherapy resistance in lung cancer.
Keywords: nipped-B-like protein, lung cancer, DNA damage response, double-strand break, autophagy