108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
基于实际和循证评估来重新评估舒血宁注射液的上市后安全性
Authors Wang C, Shi QP, Ding F, Jiang XD, Tang W, Yu ML, Zhu JH
Received 3 November 2017
Accepted for publication 7 February 2018
Published 5 April 2018 Volume 2018:12 Pages 757—767
DOI https://doi.org/10.2147/DDDT.S156000
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Palas Chanda
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Aim: To evaluate the
factors influencing suspected hypersensitivity and adverse systemic reactions
after Shuxuening injection and to provide innovative ideas and methods for the
reevaluation of post-marketing safety of Shuxuening.
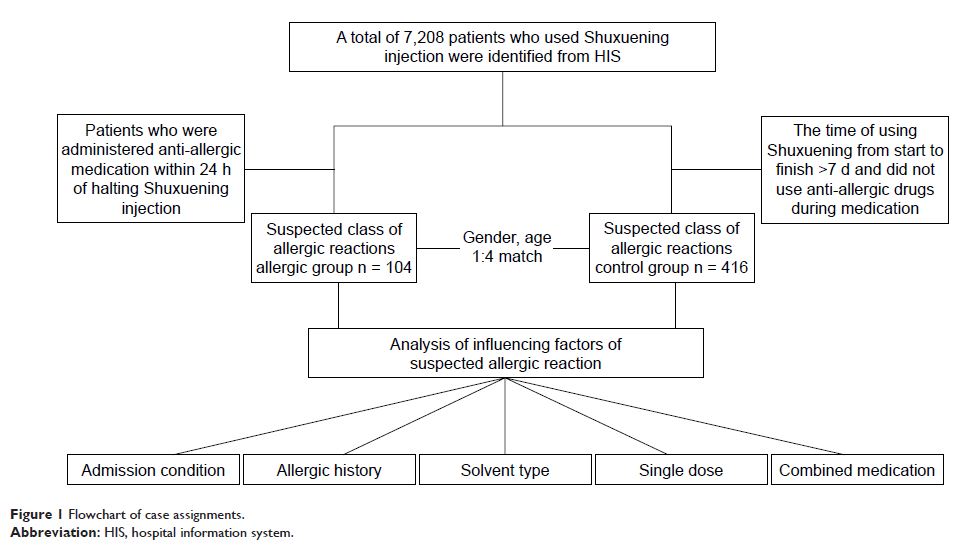
Methods: This study used a prospective, nested case–control study design, combined with a prescription sequence analysis design method. It classified patients who exhibited trigger signals after administration of Shuxuening injection as suspected allergic patients and made comparisons with patients who did not report adverse effects to calculate the correlation between relevant risk factors and suspected allergic reactions. Randomized controlled studies and cohort studies of the adverse drug reaction (ADR) of Shuxuening were performed using a computer database. Data retrieval was carried out by the foundation governing the individual database. Meta-analysis was performed by using R3.2.3 software to evaluate the ADRs of Shuxuening.
Results: The results of real-world study showed that administration of Shuxuening in combination with potassium aspartate and magnesium, atorvastatin calcium, Shengmai injection, pantoprazole sodium, or high-dose medication was a risk factor for suspected allergic reactions. Meta-analysis showed that the incidence of adverse events was 5.84% (95% CI 0.0499; 0.0674), and serious adverse reaction rate was 4.36% (95% CI 0.0188; 0.0760) when Shuxuening was used in combination with these drugs. The incidence of allergic reaction was also influenced by the vehicle, duration of treatment, single dose, and indicated vs off-label use.
Conclusion: Risk factors for adverse reaction following the use of Shuxuening injection in patients are associated with a single dose, vehicle, type of disease, and combination with potassium aspartate, atorvastatin calcium, Shengmai injection, injection with pantoprazole sodium, and other drugs. Physicians should be careful to follow guidelines when administering this drug. We further propose that the unique methodology used in this study may be useful for reevaluation of the safety of other traditional Chinese medicines.
Keywords: real-world study, evidence-based evaluation, Shuxuening injection, post-marketing safety reevaluation, allergic reaction
Methods: This study used a prospective, nested case–control study design, combined with a prescription sequence analysis design method. It classified patients who exhibited trigger signals after administration of Shuxuening injection as suspected allergic patients and made comparisons with patients who did not report adverse effects to calculate the correlation between relevant risk factors and suspected allergic reactions. Randomized controlled studies and cohort studies of the adverse drug reaction (ADR) of Shuxuening were performed using a computer database. Data retrieval was carried out by the foundation governing the individual database. Meta-analysis was performed by using R3.2.3 software to evaluate the ADRs of Shuxuening.
Results: The results of real-world study showed that administration of Shuxuening in combination with potassium aspartate and magnesium, atorvastatin calcium, Shengmai injection, pantoprazole sodium, or high-dose medication was a risk factor for suspected allergic reactions. Meta-analysis showed that the incidence of adverse events was 5.84% (95% CI 0.0499; 0.0674), and serious adverse reaction rate was 4.36% (95% CI 0.0188; 0.0760) when Shuxuening was used in combination with these drugs. The incidence of allergic reaction was also influenced by the vehicle, duration of treatment, single dose, and indicated vs off-label use.
Conclusion: Risk factors for adverse reaction following the use of Shuxuening injection in patients are associated with a single dose, vehicle, type of disease, and combination with potassium aspartate, atorvastatin calcium, Shengmai injection, injection with pantoprazole sodium, and other drugs. Physicians should be careful to follow guidelines when administering this drug. We further propose that the unique methodology used in this study may be useful for reevaluation of the safety of other traditional Chinese medicines.
Keywords: real-world study, evidence-based evaluation, Shuxuening injection, post-marketing safety reevaluation, allergic reaction