108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

AA-PMe 对 HUVEC(体外)和斑马鱼(体内)的抗血管生成作用
Authors Jing Y, Wang G, Xiao Q, Zhou Y, Wei Y, Gong Z
Received 22 November 2017
Accepted for publication 10 February 2018
Published 4 April 2018 Volume 2018:11 Pages 1871—1884
DOI https://doi.org/10.2147/OTT.S157747
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Abstract: Angiogenesis plays a vital role in many physiological and pathological
processes and several diseases are connected with its dysregulation. Asiatic
acid (AA) has demonstrated anticancer properties and we suspect this might be
attributable to an effect on angiogenesis. A modified derivative of AA,
N-(2α,3β,23-acetoxyurs-12-en-28-oyl)-L-proline methyl ester (AA-PMe), has
improved efficacy over its parent compound, but its effect on blood vessel
development remains unclear.
Methods: In this study, we investigated the antiangiogenic activity of AA and
AA-PMe in zebrafish embryos and human umbilical vein endothelial cells
(HUVECs). First of all, we treated HUVECs with increasing concentrations of
AA-PMe or AA, with or without vascular endothelial growth factor (VEGF)
present, and assessed cell viability, tube formation, and cell migration and
invasion. Quantitative real-time polymerase chain reaction and Western blot
analysis were later used to determine the role of vascular endothelial growth
factor receptor 2 (VEGFR2)-mediated signaling in AA-PMe inhibition of
angiogenesis. We extended these studies to follow angiogenesis using Tg(fli:EGFP) transgenic
zebrafish embryos. For these experiments, embryos were treated with varying
concentrations of AA-PMe or AA from 24 to 72 hours postfertilization prior to
morphological observation, angiogenesis assessment, and endogenous alkaline
phosphatase assay. VEGFR2 expression
in whole embryos following AA-PMe treatment was also determined.
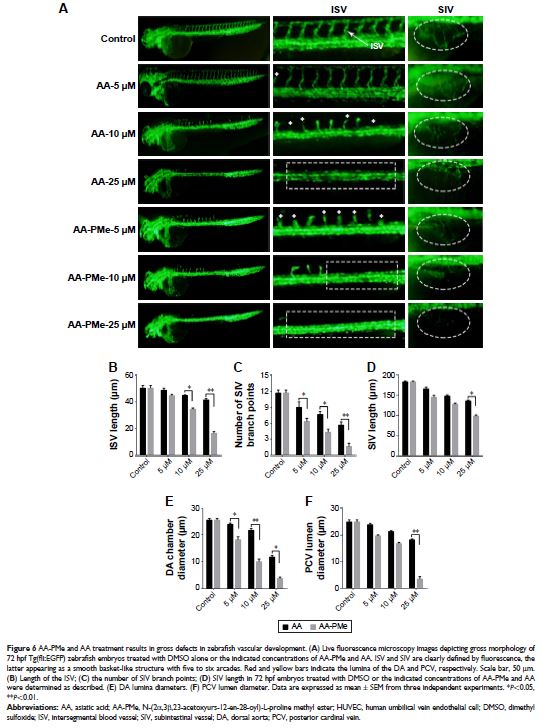
Results: We found AA-PMe decreased cell viability and inhibited migration and
tube formation in a dose-dependent manner in HUVECs. Similarly, AA-PMe
disrupted the formation of intersegmental vessels, the dorsal aorta, and the
posterior cardinal vein in zebrafish embryos. Both in vitro and in vivo AA-PMe
surpassed AA in its ability to block angiogenesis by suppressing VEGF-induced
phosphorylation of VEGFR2 and disrupting downstream extracellular regulated
protein kinase and AKT signaling.
Conclusion: For the first time, this study reveals that AA-PMe acts as a
potent VEGFR2 kinase inhibitor and exerts powerful antiangiogenic activity,
suggesting it to be a promising therapeutic candidate for further research.
Keywords: AA-PMe, angiogenesis inhibitor, zebrafish, HUVEC