108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

通过上调脑源性神经营养因子的表达,脊髓中的 Sonic hedgehog 信号传导有助于由吗啡诱导的痛觉过敏和耐受性
Authors Liu S, Yao J, Wan X, Song Z, Miao S, Zhao Y, Wang X, Liu Y
Received 9 October 2017
Accepted for publication 23 December 2017
Published 3 April 2018 Volume 2018:11 Pages 649—659
DOI https://doi.org/10.2147/JPR.S153544
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Minal Joshi
Peer reviewer comments 2
Editor who approved publication: Dr E. Alfonso Romero-Sandoval
Purpose: Preventing opioid-induced hyperalgesia and tolerance continues to
be a major clinical challenge, and the underlying mechanisms of hyperalgesia
and tolerance remain elusive. Here, we investigated the role of sonic hedgehog
(Shh) signaling in opioid-induced hyperalgesia and tolerance.
Methods: Shh signaling expression, behavioral changes, and neurochemical
alterations induced by morphine were analyzed in male adult CD-1 mice with
repeated administration of morphine. To investigate the contribution of Shh to
morphine-induced hyperalgesia (MIH) and tolerance, Shh signaling inhibitor
cyclopamine and Shh small interfering RNA (siRNA) were used. To explore the
mechanisms of Shh signaling in MIH and tolerance, brain-derived neurotrophic
factor (BDNF) inhibitor K252 and anti-BDNF antibody were used.
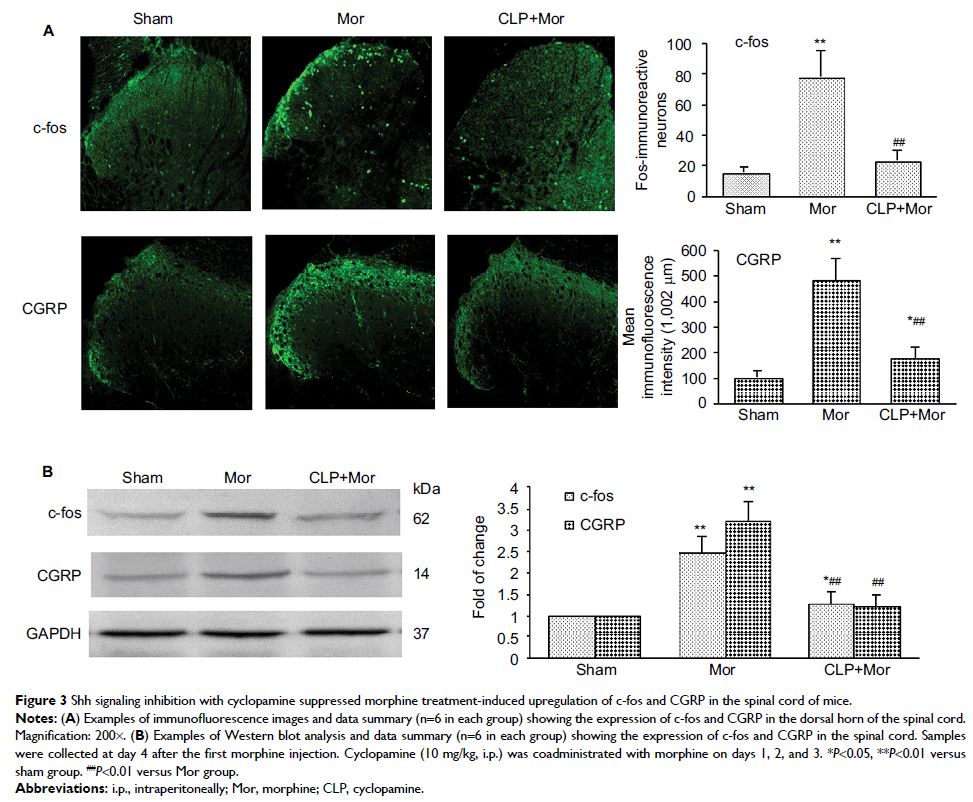
Results: Repeated administration of morphine produced obvious hyperalgesia
and tolerance. The behavioral changes were correlated with the upregulation and
activation of morphine treatment-induced Shh signaling. Pharmacologic and
genetic inhibition of Shh signaling significantly delayed the generation of MIH
and tolerance and associated neurochemical changes. Chronic morphine
administration also induced upregulation of BDNF. Inhibiting BDNF effectively
delayed the generation of MIH and tolerance. The upregulation of BDNF induced
by morphine was significantly suppressed by inhibiting Shh signaling. In naïve
mice, exogenous activation of Shh signaling caused a rapid increase of BDNF
expression, as well as thermal hyperalgesia. Inhibiting BDNF significantly
suppressed smoothened agonist-induced hyperalgesia.
Conclusion: These findings suggest that Shh signaling may be a critical mediator for
MIH and tolerance by regulating BDNF expression. Inhibiting Shh signaling,
especially during the early phase, may effectively delay or suppress MIH and
tolerance.
Keywords: sonic hedgehog, tolerance, hyperalgesia, brain-derived neurotrophic
factor, spinal cord