108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
基于表面活性剂的纳米粒子加载阿霉素以克服癌症中的多药抗药性
Authors Huang W, Lang Y, Hakeem Abdul, Lei Y, Gan L, Yang X
Received 17 November 2017
Accepted for publication 24 January 2018
Published 21 March 2018 Volume 2018:13 Pages 1723—1736
DOI https://doi.org/10.2147/IJN.S157368
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Govarthanan Muthusamy
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: Multidrug resistance
(MDR) is one of the major obstacles to successful cancer chemotherapy.
Developing efficient strategies to reverse MDR remains a major challenge.
Surfactin (SUR), a cyclic lipopeptide biosurfactant, has been found to display
anticancer activity.
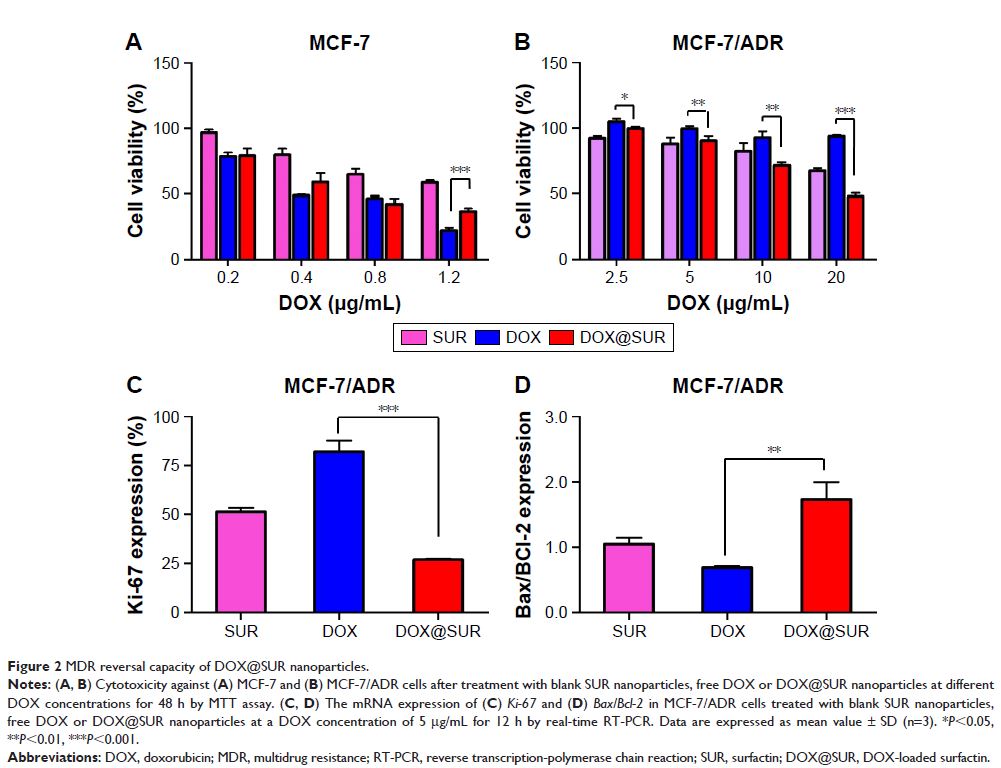
Methods: In this paper, SUR was assembled by solvent-emulsion method to load the anticancer drug doxorubicin (DOX). The cytotoxicity of DOX-loaded SUR nanoparticles (DOX@SUR) against DOX-resistant human breast cancer MCF-7/ADR is measured by MTT assay. The cellular uptake and intracellular retention of DOX@SUR are determined by flow cytometry. The tumor accumulation and anticancer activity of DOX@SUR are evaluated in MCF-7/ADR-bearing nude mice.
Results: DOX@SUR induce stronger cytotoxicity against DOX-resistant human breast cancer MCF-7/ADR cells compared to free DOX. DOX@SUR nanoparticles exhibit enhanced cellular uptake and decreased cellular efflux, which might be associated with reduced P-glycoprotein expression. After internalization into MCF-7/ADR cells by macropinocytosis- and caveolin-mediated endocytosis, DOX@SUR nanoparticles are colocalized with the lysosomes and translocated to the nucleus to exert cytotoxicity. Furthermore, in vivo animal experiment shows that the DOX@ SUR nanoparticles are accumulated more efficiently in tumors than free DOX. Meanwhile, DOX@SUR nanoparticles display stronger tumor inhibition activity and fewer side effects in MCF-7/ADR-bearing nude mice.
Conclusion: This study indicates that SUR-based nanocarrier might present a promising platform to reverse MDR in cancer chemotherapy.
Keywords: multidrug resistance, cellular uptake, cellular efflux, biodistribution
Methods: In this paper, SUR was assembled by solvent-emulsion method to load the anticancer drug doxorubicin (DOX). The cytotoxicity of DOX-loaded SUR nanoparticles (DOX@SUR) against DOX-resistant human breast cancer MCF-7/ADR is measured by MTT assay. The cellular uptake and intracellular retention of DOX@SUR are determined by flow cytometry. The tumor accumulation and anticancer activity of DOX@SUR are evaluated in MCF-7/ADR-bearing nude mice.
Results: DOX@SUR induce stronger cytotoxicity against DOX-resistant human breast cancer MCF-7/ADR cells compared to free DOX. DOX@SUR nanoparticles exhibit enhanced cellular uptake and decreased cellular efflux, which might be associated with reduced P-glycoprotein expression. After internalization into MCF-7/ADR cells by macropinocytosis- and caveolin-mediated endocytosis, DOX@SUR nanoparticles are colocalized with the lysosomes and translocated to the nucleus to exert cytotoxicity. Furthermore, in vivo animal experiment shows that the DOX@ SUR nanoparticles are accumulated more efficiently in tumors than free DOX. Meanwhile, DOX@SUR nanoparticles display stronger tumor inhibition activity and fewer side effects in MCF-7/ADR-bearing nude mice.
Conclusion: This study indicates that SUR-based nanocarrier might present a promising platform to reverse MDR in cancer chemotherapy.
Keywords: multidrug resistance, cellular uptake, cellular efflux, biodistribution