108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
用透明质酸修饰的加载 siRNA 的硒纳米颗粒用于加强肝细胞癌的治疗效果
Authors Xia Y, Guo M, Xu TT, Li YH, Wang CB, Lin ZF, Zhao MQ, Zhu B
Received 19 November 2017
Accepted for publication 24 January 2018
Published 15 March 2018 Volume 2018:13 Pages 1539—1552
DOI https://doi.org/10.2147/IJN.S157519
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Govarthanan Muthusamy
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Background: Small interfering RNA
(siRNA) as a new therapeutic modality holds promise for cancer treatment.
However, the traditional viral carriers are prone to immunogenicity and risk of
insertional mutagenesis.
Methods: In order to provide a tumor-targeted delivery carrier of siRNA in cancer therapy, the hyaluronic acid (HA)-selenium (Se)-polyethylenimine (PEI) nanoparticle (NP) was fabricated by decorating SeNP with HA as a tumor-targeting moiety and by linking the polycationic polymers polyethylenimine PEI onto the surface of SeNP. The siRNA was loaded to the surface of SeNP HA-Se-PEI via the electrostatic interaction between siRNA and PEI to prepare the functionalized SeNP HA-Se-PEI@siRNA.
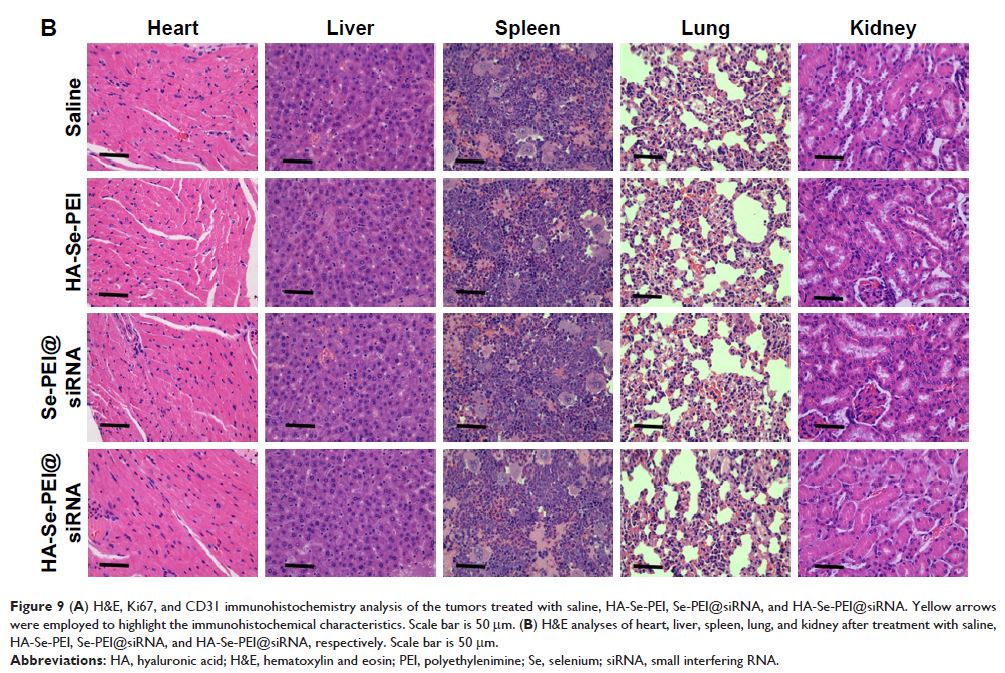
Results: The HA-Se-PEI@siRNA was internalized into the HepG2 cell mainly in a clathrin-mediated endocytosis manner. Owing to the active tumor-targeted effect mediated by HA, HA-Se-PEI@siRNA achieved the obvious higher transfection efficiency, greater gene silencing ability, and stronger cytotoxicity in the HepG2 cell compared with the passive tumor-targeted NP Se-PEI@siRNA. The knockdown of hairy and enhancer of split 5 by HA-Se-PEI@siRNA induced the HepG2 cell cycle arrest at the G0/G1 phase and apoptosis. Furthermore, the treatment with HA-Se-PEI@siRNA resulted in greater antitumor efficacy compared with the Se-PEI@siRNA in vitro and in vivo. In addition, the HA-Se-PEI@siRNA was almost no toxic to the key organs of mice.
Conclusion: These findings provided an alternative therapeutic route for targeted cancer treatments.
Keywords: hepatocellular carcinoma, functionalized nanoparticle, siRNA delivery, gene therapy, tumor targeting
Methods: In order to provide a tumor-targeted delivery carrier of siRNA in cancer therapy, the hyaluronic acid (HA)-selenium (Se)-polyethylenimine (PEI) nanoparticle (NP) was fabricated by decorating SeNP with HA as a tumor-targeting moiety and by linking the polycationic polymers polyethylenimine PEI onto the surface of SeNP. The siRNA was loaded to the surface of SeNP HA-Se-PEI via the electrostatic interaction between siRNA and PEI to prepare the functionalized SeNP HA-Se-PEI@siRNA.
Results: The HA-Se-PEI@siRNA was internalized into the HepG2 cell mainly in a clathrin-mediated endocytosis manner. Owing to the active tumor-targeted effect mediated by HA, HA-Se-PEI@siRNA achieved the obvious higher transfection efficiency, greater gene silencing ability, and stronger cytotoxicity in the HepG2 cell compared with the passive tumor-targeted NP Se-PEI@siRNA. The knockdown of hairy and enhancer of split 5 by HA-Se-PEI@siRNA induced the HepG2 cell cycle arrest at the G0/G1 phase and apoptosis. Furthermore, the treatment with HA-Se-PEI@siRNA resulted in greater antitumor efficacy compared with the Se-PEI@siRNA in vitro and in vivo. In addition, the HA-Se-PEI@siRNA was almost no toxic to the key organs of mice.
Conclusion: These findings provided an alternative therapeutic route for targeted cancer treatments.
Keywords: hepatocellular carcinoma, functionalized nanoparticle, siRNA delivery, gene therapy, tumor targeting