108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
关于左乙拉西坦治疗小儿癫痫患者随机对照试验的荟萃分析
Authors Zhang LL, Wang CZ, Li W
Received 11 September 2017
Accepted for publication 2 February 2018
Published 14 March 2018 Volume 2018:14 Pages 769—779
DOI https://doi.org/10.2147/NDT.S151413
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Objective: To evaluate clinical
efficacy, safety, and tolerability of levetiracetam as mono- or adjunctive
therapy in the treatment of children and adolescents with epilepsy.
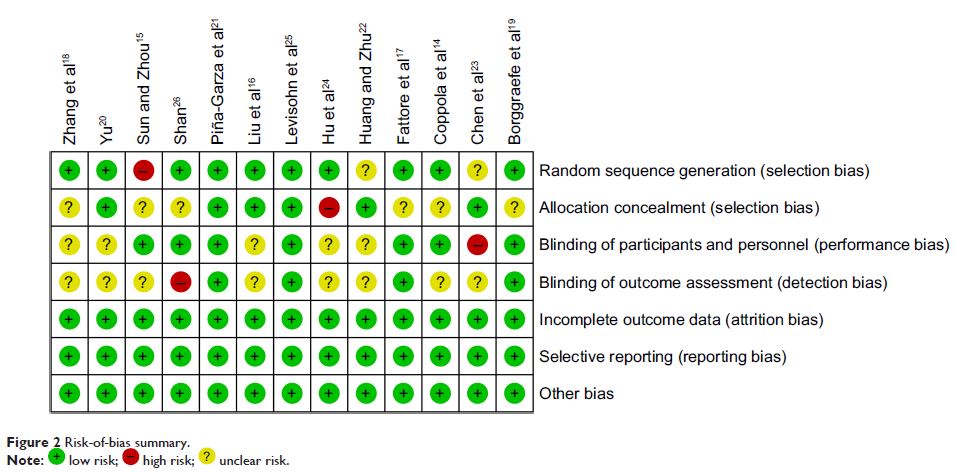
Materials and methods: We performed a meta-analysis of randomized controlled trials published from January 2007 to December 2016 in the databases Web of Science, Medline, Embase, Cochrane Library, and PubMed, Bing, Baidu, Google Scholar, Chinese National Knowledge Infrastructure (CNKI), and Wanfang Data. All of the studies eligible were compared for the efficacy, safety, and tolerability of levetiracetam with other antiepileptic drugs (AEDs) in epilepsy.
Results: Thirteen randomized controlled trials on a total of 1,013 patients met the inclusion criteria in present study. Compared with other AEDs (oxcarbazepine, valproate, sulthiame, carbamazepine, and placebo), we found that levetiracetam had a comparable seizure-free rate (RR 1.16, 95% CI 1.03–1.31; P =0.30). Regarding seizure-frequency reduction ≥50% from baseline, levetiracetam also seemed equivalent to other AEDs (RR 1.08, 95% CI 1.01–1.16; P =0.35). In spite of patients treated with levetiracetam having a lower incidence of side effects compared with patients treated with other AEDs (RR 0.90, 95% CI 0.77–1.06), the difference between them was minute and not statistically significant (P =0.22).
Conclusion: Based on this meta-analysis, it seemed that levetiracetam had comparable effects concerning efficacy, tolerability, and adverse events. Nevertheless, 13 studies were insufficient to draw a conclusion that levetiracetam is effective as mono- and adjunctive therapy for all types of epilepsy syndromes and seizures. Larger-sample and more well-designed trials are needed to justify the widespread use of levetiracetam in the treatment of children and adolescents.
Keywords: levetiracetam, epilepsy, children, antiepileptic drug, RCT, efficacy
Materials and methods: We performed a meta-analysis of randomized controlled trials published from January 2007 to December 2016 in the databases Web of Science, Medline, Embase, Cochrane Library, and PubMed, Bing, Baidu, Google Scholar, Chinese National Knowledge Infrastructure (CNKI), and Wanfang Data. All of the studies eligible were compared for the efficacy, safety, and tolerability of levetiracetam with other antiepileptic drugs (AEDs) in epilepsy.
Results: Thirteen randomized controlled trials on a total of 1,013 patients met the inclusion criteria in present study. Compared with other AEDs (oxcarbazepine, valproate, sulthiame, carbamazepine, and placebo), we found that levetiracetam had a comparable seizure-free rate (RR 1.16, 95% CI 1.03–1.31; P =0.30). Regarding seizure-frequency reduction ≥50% from baseline, levetiracetam also seemed equivalent to other AEDs (RR 1.08, 95% CI 1.01–1.16; P =0.35). In spite of patients treated with levetiracetam having a lower incidence of side effects compared with patients treated with other AEDs (RR 0.90, 95% CI 0.77–1.06), the difference between them was minute and not statistically significant (P =0.22).
Conclusion: Based on this meta-analysis, it seemed that levetiracetam had comparable effects concerning efficacy, tolerability, and adverse events. Nevertheless, 13 studies were insufficient to draw a conclusion that levetiracetam is effective as mono- and adjunctive therapy for all types of epilepsy syndromes and seizures. Larger-sample and more well-designed trials are needed to justify the widespread use of levetiracetam in the treatment of children and adolescents.
Keywords: levetiracetam, epilepsy, children, antiepileptic drug, RCT, efficacy