108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

细胞穿透/肿瘤靶向肽修饰的纳米载体用于肿瘤靶向递送草药类药物
Authors Kebebe D, Liu Y, Wu Y, Vilakhamxay M, Liu Z, Li J
Received 9 November 2017
Accepted for publication 24 January 2018
Published 9 March 2018 Volume 2018:13 Pages 1425—1442
DOI https://doi.org/10.2147/IJN.S156616
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
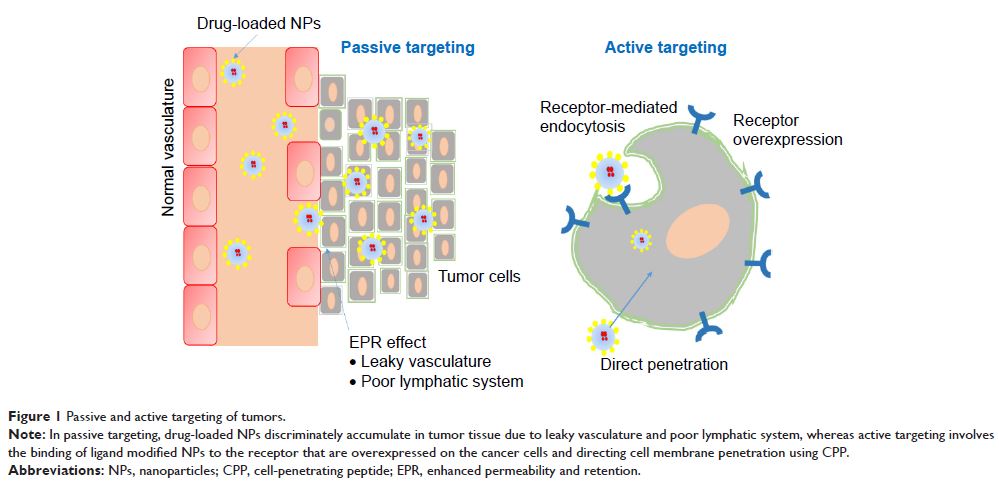
Abstract: Cancer has become one of the
leading causes of mortality globally. The major challenges of conventional
cancer therapy are the failure of most chemotherapeutic agents to accumulate
selectively in tumor cells and their severe systemic side effects. In the past
three decades, a number of drug delivery approaches have been discovered to
overwhelm the obstacles. Among these, nanocarriers have gained much attention
for their excellent and efficient drug delivery systems to improve specific
tissue/organ/cell targeting. In order to enhance targeting efficiency further
and reduce limitations of nanocarriers, nanoparticle surfaces are
functionalized with different ligands. Several kinds of ligand-modified
nanomedicines have been reported. Cell-penetrating peptides (CPPs) are
promising ligands, attracting the attention of researchers due to their
efficiency to transport bioactive molecules intracellularly. However, their
lack of specificity and in vivo degradation led to the development of newer
types of CPP. Currently, activable CPP and tumor-targeting peptide
(TTP)-modified nanocarriers have shown dramatically superior cellular specific
uptake, cytotoxicity, and tumor growth inhibition. In this review, we discuss
recent advances in tumor-targeting strategies using CPPs and their limitations
in tumor delivery systems. Special emphasis is given to activable CPPs and
TTPs. Finally, we address the application of CPPs and/or TTPs in the delivery
of plant-derived chemotherapeutic agents.
Keywords: cancer,
nanocarriers, cell-penetrating peptide, targeting drug delivery, herb-based
drug, tumor targeting