108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

在剂量调查临床试验中的样本量:系统评估
Authors Huang JH, Su QM, Yang J, Lv YH, He YC, Chen JC, Xu L, Wang K, Zheng QS
Published Date January 2015 Volume 2015:9 Pages 305—312
DOI http://dx.doi.org/10.2147/DDDT.S76135
Received 21 October 2014, Accepted 2 December 2014, Published 7 January 2015
Abstract: The main
purpose of investigational phase II clinical trials is to explore indications
and effective doses. However, as yet, there is no clear rule and no related
published literature about the precise suitable sample sizes to be used in
phase II clinical trials. To explore this, we searched for clinical trials in
the ClinicalTrials.gov registry using the keywords “dose-finding” or
“dose–response” and “Phase II”. The time span of the search was September 20,
1999, to December 31, 2013. A total of 2103 clinical trials were finally included in our review.
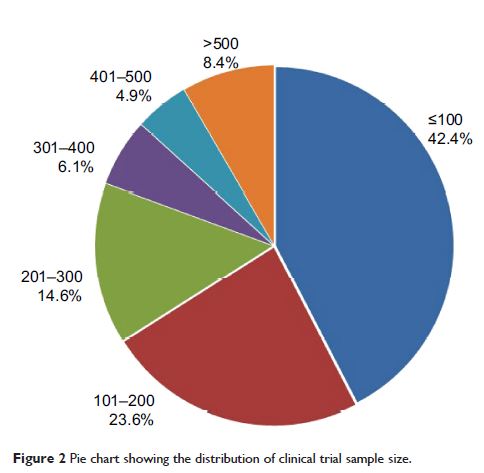
Regarding sample sizes, 1,156 clinical trials had <40 participants
in each group, accounting for 55.0% of the studies reviewed, and only 17.2% of
the studies reviewed had >100 patient cases in a single group. Sample
sizes used in parallel study designs tended to be larger than those of
crossover designs (median sample size 151 and 37, respectively). In
conclusion, in the earlier phases of drug research and development, there are a
variety of designs for dosage investigational studies. The sample size of each
trial should be comprehensively considered and selected according to the study
design and purpose.
Keywords: sample number,
dose-finding, dose–response, systematic review