108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

HepG2 细胞中外切体介导的工程化功能活性 miR-26a 的递送及其增强的抑制作用
Authors Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S
Received 18 October 2017
Accepted for publication 7 December 2017
Published 30 January 2018 Volume 2018:13 Pages 585—599
DOI https://doi.org/10.2147/IJN.S154458
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Jiang Yang
Peer reviewer comments 5
Editor who approved publication: Dr Linlin Sun
Introduction: Exosomes
are closed-membrane nanovesicles that are secreted by a variety of cells and
exist in most body fluids. Recent studies have demonstrated the potential of
exosomes as natural vehicles that target delivery of functional small RNA and
chemotherapeutics to diseased cells.
Methods: In this study, we introduce a new approach for the targeted
delivery of exosomes loaded with functional miR-26a to scavenger receptor class
B type 1-expressing liver cancer cells. The tumor cell-targeting function of
these engineered exosomes was introduced by expressing in 293T cell hosts, the
gene fusion between the transmembrane protein of CD63 and a sequence from
Apo-A1. The exosomes harvested from these 293T cells were loaded with miR-26a
via electroporation.
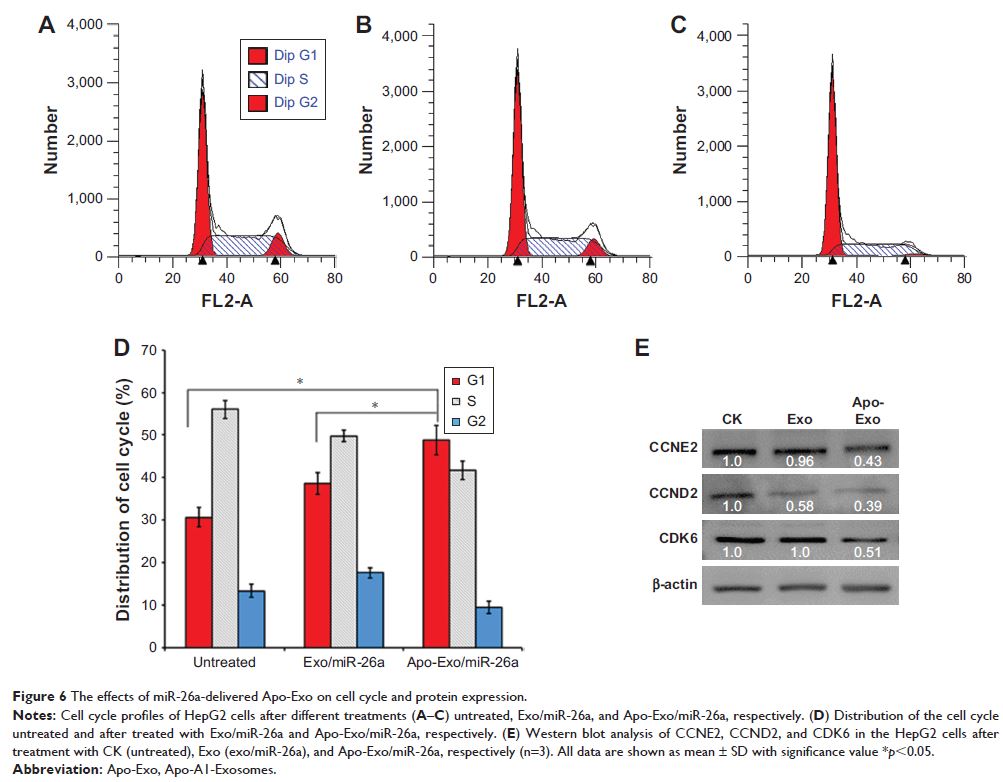
Results: The engineered exosomes were shown to bind selectively to HepG2 cells
via the scavenger receptor class B type 1–Apo-A1 complex and then internalized
by receptor-mediated endocytosis. The release of miR-26a in exosome-treated
HepG2 cells upregulated miR-26a expression and decreased the rates of cell
migration and proliferation. We also presented evidence that suggest cell
growth was inhibited by miR-26a-mediated decreases in the amounts of key
proteins that regulate the cell cycle.
Conclusion: Our gene delivery strategy can be adapted to treat a broad
spectrum of cancers by expressing proteins on the surface of miRNA-loaded
exosomes that recognize specific biomarkers on the tumor cell.
Keywords: exosome, gene delivery, miR-26a, HepG2 cells