108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

用于测量 NSCLC 患者血浆 EGFR T790M 突变的 QuantStudio™ 3D 数字 PCR 和 ARMS-PCR 的比较
Authors Feng Q, Gai F, Sang Y, Zhang J, Wang P, Wang Y, Liu B, Lin D, Yu Y, Fang J
Received 2 August 2017
Accepted for publication 31 October 2017
Published 18 January 2018 Volume 2018:10 Pages 115—121
DOI https://doi.org/10.2147/CMAR.S148134
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Background: The AURA3 clinical trial has shown that advanced non-small cell lung
cancer (NSCLC) patients with EGFR T790M mutations in circulating tumor DNA
(ctDNA) could benefit from osimertinib.
Purpose: The aim of this study was to assess the
usefulness of QuantStudio™ 3D Digital PCR System platform for the detection of
plasma EGFR T790M mutations in NSCLC patients, and compare the performances of
3D Digital PCR and ARMS-PCR.
Patients and methods: A total of 119 Chinese patients were enrolled in this
study. Mutant allele frequency of plasma EGFR T790M was detected by 3D Digital
PCR, then 25 selected samples were verified by ARMS-PCR and four of them were
verified by next generation sequencing (NGS).
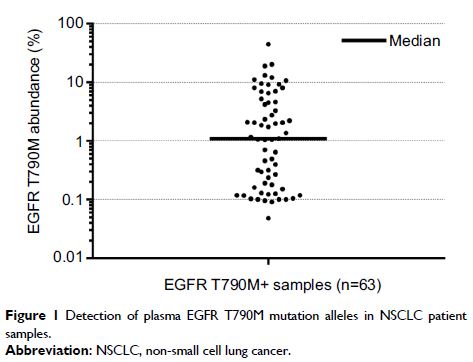
Results: In total, 52.94% (69/119) had EGFR T790M
mutations detected by 3D Digital PCR. In 69 positive samples, the median mutant
allele frequency (AF) was 1.09% and three cases presented low concentration (AF
<0.1%). Limited by the amount of plasma DNA, 17 samples (AF <2.5%) and
eight samples (T790M-) were selected for verification by ARMS-PCR. Four of
those samples were verified by NGS as a third verification method. Among the
selected 17 positive cases, ten samples presented mutant allele frequency
<0.5%, and seven samples presented intermediate mutant allele frequency
(0.5%<AF<2.5%). However, only three samples (3/17) were identified as
positive by ARMS-PCR, namely, P6 (AF =1.09%), P7 (AF =2.09%), and P8 (AF
=2.21%). It is worth mentioning that sample P9 (AF =2.05%, analyzed by 3D
Digital PCR) was identified as T790M- by ARMS-PCR. Four samples were identified
as T790M+ by both NGS and 3D Digital PCR, and typically three samples (3/4)
presented at a low ratio (AF <0.5%).
Conclusion: Our study demonstrated that 3D Digital PCR is a novel
method with high sensitivity and specificity to detect EGFR T790M mutation in
plasma.
Keywords: 3D Digital PCR,
allele frequency, EGFR TKIs, resistance, osimertinib, erlotinib, gefitinib,
icotinib