108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

载有 miRNA-135a 的新型纳米载体对恶性胶质瘤的抗肿瘤作用
Authors Liang C, Sun W, He H, Zhang B, Ling C, Wang B, Huang T, Hou B, Guo Y
Received 2 August 2017
Accepted for publication 13 November 2017
Published 29 December 2017 Volume 2018:13 Pages 209—220
DOI https://doi.org/10.2147/IJN.S148142
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Yang Liu
Peer reviewer comments 2
Editor who approved publication: Dr Lei Yang
Introduction: MiR-135a is found to selectively induce apoptosis in glioma cell
but not in normal neurons and glial cells. However, low transfection efficacy
limits its application in vivo as other miRNAs. We prepared a new kind of
nano-vector based on polyethylene glycol methyl ether (mPEG) and hyper-branched
polyethylenimine (hy-PEI) in order to improve the miRNA delivery system into
the glioma cells.
Methods: The mPEG-g-PEI/miR-135a was constructed and detected
by 1H NMR and FTIR analyses. Transmission electron
microscope was utilized for its characteristics. Stability and release
efficiency was assessed by electrophoresis. Biocompatibility was observed and
analyzed through co-culture with astrocytes and malignant glioma cells (C6).
Transfection rate was monitored by laser confocal microscopy and flow
cytometry. The antitumor effect of mPEG-g-PEI/miR-135a to C6 was confirmed in
vivo by MR scanning, pathology and survival curve. RT-PCR was used to assay
transfection efficiency of mPEG-g-PEI/miR-135a in vitro and in vivo. And
Western blotting was used to assess the expressions of the targeted proteins of
miR-135a.
Results: In this experiment, we found the optimal N/P ratio of
mPEG-g-PEI/miR-135a was about 6 judged by Zeta potential, particle size and
encapsulation ability. The stability of mPEG-g-PEI/miR-135a in serum and the
release efficiency in acid(pH=5.0) of mPEG-g-PEI/miR-135a were simulated the
environment in vivo and in tumor. The mPEG-g-PEI nano-vector was co-cultured
with malignant glioma cell C6 and normal astrocytes in vitro and showed good
biocompatibility evaluated by CCK8 assay. The cell experiments in vitro
indicated that mPEG-g-PEI could significantly improve miR-135a transfection by
enhancing uptake effect of both normal glial and glioma cells. Given the C6
implanted in situ model, we discovered that the mPEG-g-PEI/miR-135a could
obviously increase the survival period and inhibit the growth of glioma
confirmed by MRI and histochemistry. In addition, the transfection efficiency
of mPEG-g-PEI was better than that of other transfection agents either in vitro
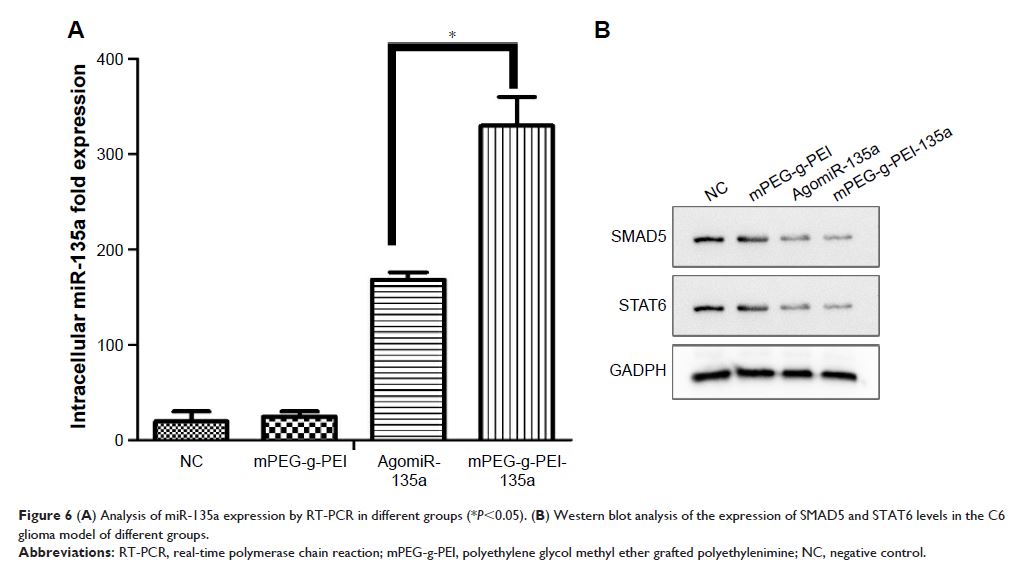
or in vivo confirmed by RT-PCR. Moreover, the expressions of the targeted
proteins of miR-135a were consistent with the in vitro results.
Conclusion: These results suggest that mPEG-g-PEI is expected to
provide a new effective intracellular miRNA delivery system with low toxicity
for glioma therapy.
Keywords: nano-vector,
miRNA-135a, malignant glioma, gene delivery, Micro RNA