108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Polyvinylpyrrolidone-coated gold nanoparticles inhibit endothelial cell viability, proliferation, and ERK1/2 phosphorylation and reduce the magnitude of endothelial-independent dilator responses in isolated aortic vessels
Authors Mohamed T, Matou-Nasri S, Farooq A, Whitehead D, Azzawi M
Received 19 March 2017
Accepted for publication 7 August 2017
Published 13 December 2017 Volume 2017:12 Pages 8813—8830
DOI https://doi.org/10.2147/IJN.S133093
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Background: Gold nanoparticles (AuNPs) demonstrate clinical potential for drug
delivery and imaging diagnostics. As AuNPs aggregate in physiological fluids,
polymer-surface modifications are utilized to allow their stabilization and
enhance their retention time in blood. However, the impact of AuNPs on blood
vessel function remains poorly understood. In the present study, we
investigated the effects of AuNPs and their stabilizers on endothelial cell
(EC) and vasodilator function.
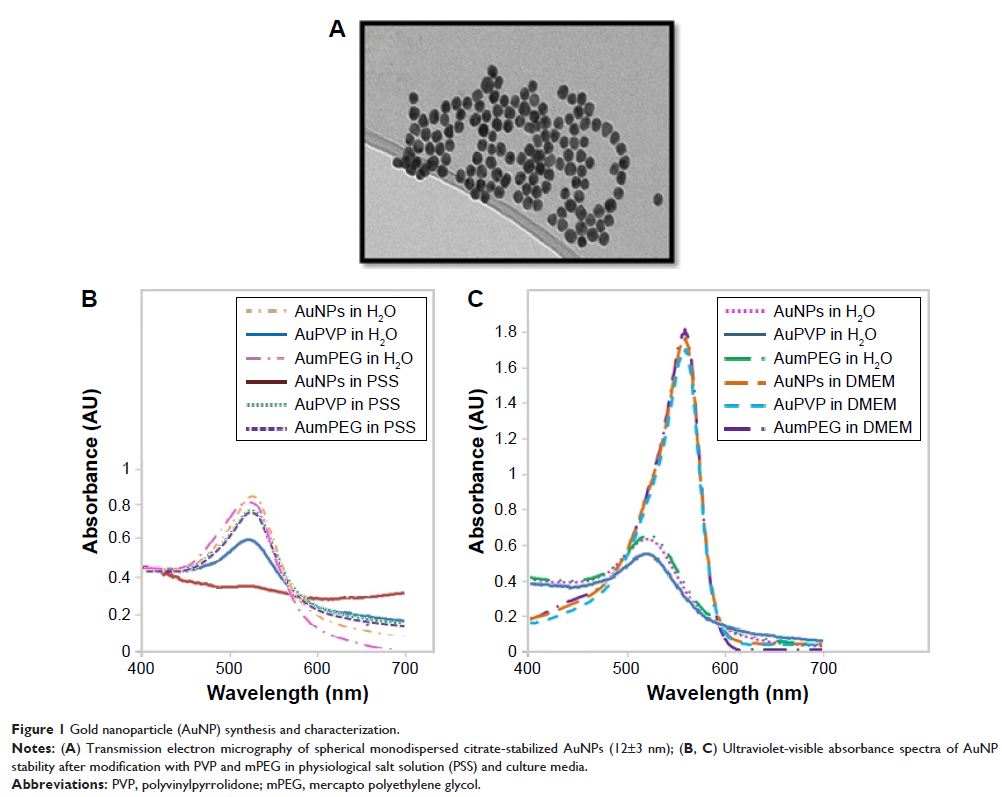
Materials and methods: Citrate-stabilized AuNPs (12±3 nm) were synthesized
and surface-modified using mercapto polyethylene glycol (mPEG) and
polyvinylpyrrolidone (PVP) polymers. Their uptake by isolated ECs and whole
vessels was visualized using transmission electron microscopy and quantified
using inductively coupled plasma mass spectrometry. Their biological effects on
EC proliferation, viability, apoptosis, and the ERK1/2-signaling pathway were
determined using automated cell counting, flow cytometry, and Western blotting,
respectively. Endothelial-dependent and independent vasodilator functions were
assessed using isolated murine aortic vessel rings ex vivo.
Results: AuNPs were located in endothelial endosomes within 30
minutes’ exposure, while their surface modification delayed this cellular
uptake over time. After 24 hours’ exposure, all AuNPs (including polymer-modified
AuNPs) induced apoptosis and decreased cell viability/proliferation. These
inhibitory effects were lost after 48 hours’ exposure (except for the
PVP-modified AuNPs). Furthermore, all AuNPs decreased acetylcholine
(ACh)-induced phosphorylation of ERK1/2, a key signaling protein of cell
function. mPEG-modified AuNPs had lower cytostatic effects than PVP-modified
AuNPs. Citrate-stabilized AuNPs did not alter endothelial-dependent
vasodilation induced by ACh, but attenuated endothelial-independent responses
induced by sodium nitroprusside. PVP-modified AuNPs attenuated ACh-induced
dilation, whereas mPEG-modified AuNPs did not, though this was dose-related.
Conclusion: We demonstrated that mPEG-modified AuNPs at a
therapeutic dosage showed lower cytostatic effects and were less detrimental to
vasodilator function than PVP-modified AuNPs, indicating greater potential as
agents for diagnostic imaging and therapy.
Keywords: nanoparticles,
gold, vascular, vasodilation, artery, cell culture