108384
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

布地奈德福莫特罗治疗 COPD 后不同高分辨率计算机断层显像的代谢变化
Authors Wang C, Li JX, Tang D, Zhang JQ, Fang LZ, Fu WP, Liu L, Dai LM
Received 20 September 2017
Accepted for publication 26 October 2017
Published 6 December 2017 Volume 2017:12 Pages 3511—3521
DOI https://doi.org/10.2147/COPD.S152134
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Charles Downs
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Chunxue Bai
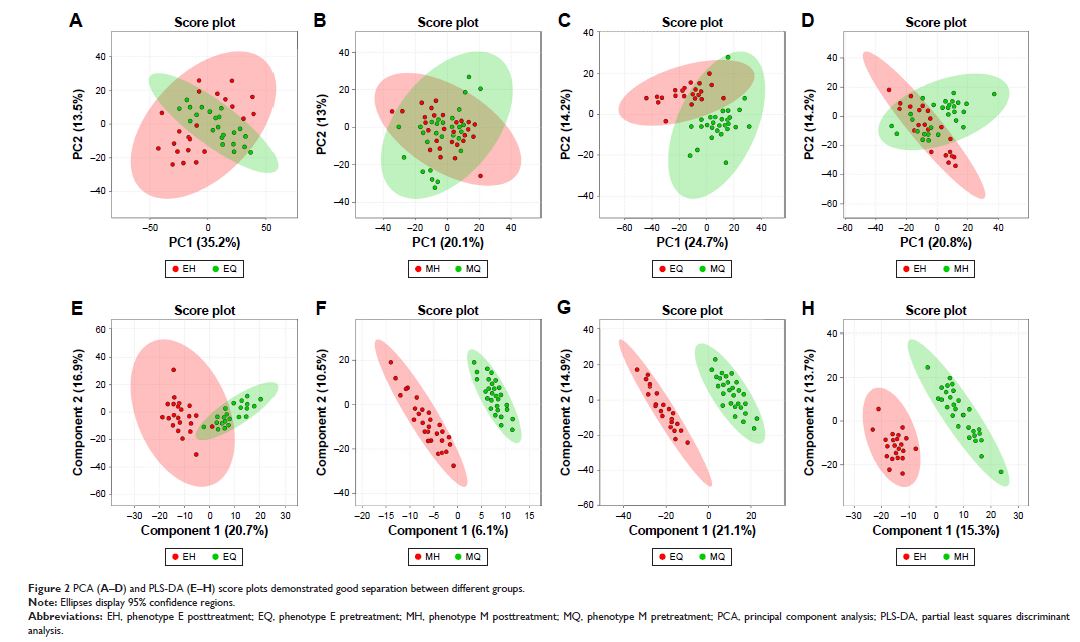
Background: Metabolomics is the global unbiased analysis of all the
small-molecule metabolites within a biological system. Metabolic profiling of
different high-resolution computed tomography (HRCT) phenotypes of COPD
patients before and after treatment may identify discriminatory metabolites
that can serve as biomarkers and therapeutic agents.
Patients and methods: 1H nuclear
magnetic resonance spectroscopy (1H-NMR)-based
metabolomics was performed on a discovery set of plasma samples from 50
patients with stable COPD. Patients were assigned into two groups on the basis
of HRCT findings including phenotype E (n=22) and phenotype M (n=28). After
budesonide–formoterol treatment (160/4.5 µg ×2 inhalations twice
daily for 3 months), clinical characteristics and metabolites were then
compared between phenotype E pretreatment and posttreatment, phenotype M
pretreatment and posttreatment, phenotype E pretreatment and phenotype M
pretreatment, and phenotype E posttreatment and phenotype M posttreatment.
Results: Inhaled budesonide–formoterol therapy for both
phenotype E (emphysema without bronchial wall thickening) and phenotype M
(emphysema with bronchial wall thickening) was effective. However, phenotype E
and phenotype M were different in response to therapy. Patients with phenotype
M in response to therapeutic effects were significantly greater compared with
phenotype E. Certain metabolites were identified, which were closely related to
the treatment and phenotype. Metabolic changes in phenotype E or phenotype M
after treatment may be involved with adenosine diphosphate (ADP), guanosine,
choline, malonate, tyrosine, glycine, proline, l-alanine, l-valine, l-threonine
leucine, uridine, pyruvic acid, acetone and metabolism disturbance. Metabolic
differences between phenotype E and phenotype M in pretreatment and
posttreatment covered glycine, D-glucose, pyruvic acid, succinate, lactate,
proline, l-valine and leucine.
Conclusion: Bronchial wall thickening in COPD may be an
indicator for predicting the better response to the treatment with
bronchodilator and corticosteroid. The identification of metabolic alterations
provides new insights into different HRCT phenotypes and therapeutic assessment
of COPD.
Keywords: COPD,
metabolomics, budesonide–formoterol, HRCT