108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

已发表论文
健康男性中国志愿者空腹和进食两种剂型马来酸氟吡汀胶囊的生物等效性研究
Authors Liu YF, Huo H, Zhao ZB, Hu WL, Sun YJ, Tang YB
Received 24 August 2017
Accepted for publication 21 October 2017
Published 1 December 2017 Volume 2017:11 Pages 3441—3448
DOI https://doi.org/10.2147/DDDT.S149913
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Akshita Wason
Peer reviewer comments 2
Editor who approved publication: Dr Anastasios Lymperopoulos
Aim: This study
developed a high-performance liquid chromatography–tandem mass spectrometry
method to simultaneously determine the concentrations of flupirtine and its
major active metabolite D-13223 in human plasma in order to assess the
bioequivalence (BE) of two flupirtine maleate capsules among healthy male
Chinese volunteers under fasting and fed conditions.
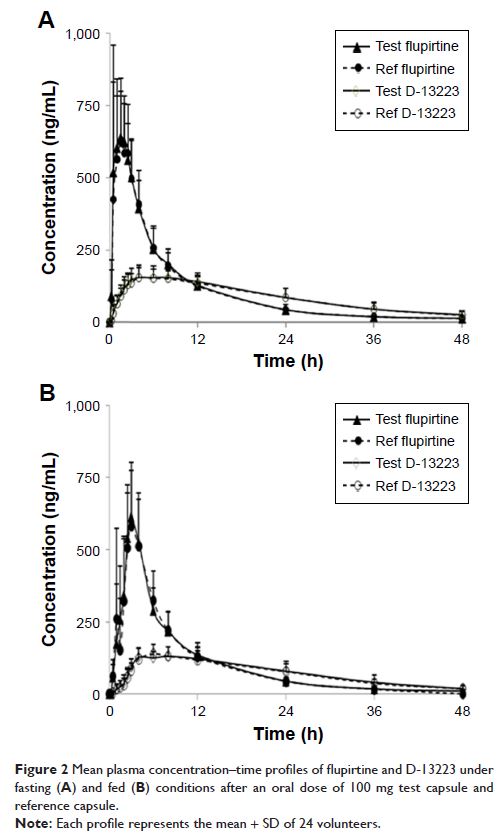
Materials and methods: There were two single-center, randomized, single-dose, open-label, laboratory-blinded, two-period, cross-over studies which included 24 healthy male Chinese volunteers under fasting and fed conditions, respectively. Plasma samples were collected prior to and up to 48 h after dosing. The concentrations of flupirtine and its major active metabolite D-13223 in plasma samples were determined by a validated method, that is, high-performance liquid chromatography coupled with a tandem mass spectrometry detector. Pharmacokinetic metrics of area from time zero to the last measurable concentration (AUC0-t), area under the plasma concentration–time curve from administration to infinite time (AUC0-∞), and Cmax were used for BE assessment.
Results: Forty-eight healthy volunteers who met the criteria were enrolled and completed the study. According to the observation of vital signs and laboratory measurement, no volunteers had any adverse reactions. Under fasting condition, the geometric mean ratios (90% CI) of the test/reference drug for flupirtine were 103.0% (98.1%–108.2%) for AUC0-t, 102.9% (98.2%–107.9%) for AUC0-∞, and 97.0% (85.9%–109.5%) for Cmax. Under fed condition, the geometric mean ratios (90% CI) of the test/reference drug for flupirtine were 101.7% (98.4%–105.1%) for AUC0-t, 101.6% (98.5%–104.8%) for AUC0-∞, and 103.5% (94.7%–113.0%) for Cmax. The difference between test and reference formulations, Tmax, was not statistically significant. The 90% CIs of the test/reference AUC ratio and Cmax ratio of D-13223 were also within the acceptance range for BE both under fasting and fed conditions.
Conclusion: The two formulations of flupirtine maleate capsule were bioequivalent (the test and the reference products) under fasting and fed conditions, and thus both can be used interchangeably in the clinical setting.
Keywords: flupirtine, D-13223, LC–MS/MS
Materials and methods: There were two single-center, randomized, single-dose, open-label, laboratory-blinded, two-period, cross-over studies which included 24 healthy male Chinese volunteers under fasting and fed conditions, respectively. Plasma samples were collected prior to and up to 48 h after dosing. The concentrations of flupirtine and its major active metabolite D-13223 in plasma samples were determined by a validated method, that is, high-performance liquid chromatography coupled with a tandem mass spectrometry detector. Pharmacokinetic metrics of area from time zero to the last measurable concentration (AUC0-t), area under the plasma concentration–time curve from administration to infinite time (AUC0-∞), and Cmax were used for BE assessment.
Results: Forty-eight healthy volunteers who met the criteria were enrolled and completed the study. According to the observation of vital signs and laboratory measurement, no volunteers had any adverse reactions. Under fasting condition, the geometric mean ratios (90% CI) of the test/reference drug for flupirtine were 103.0% (98.1%–108.2%) for AUC0-t, 102.9% (98.2%–107.9%) for AUC0-∞, and 97.0% (85.9%–109.5%) for Cmax. Under fed condition, the geometric mean ratios (90% CI) of the test/reference drug for flupirtine were 101.7% (98.4%–105.1%) for AUC0-t, 101.6% (98.5%–104.8%) for AUC0-∞, and 103.5% (94.7%–113.0%) for Cmax. The difference between test and reference formulations, Tmax, was not statistically significant. The 90% CIs of the test/reference AUC ratio and Cmax ratio of D-13223 were also within the acceptance range for BE both under fasting and fed conditions.
Conclusion: The two formulations of flupirtine maleate capsule were bioequivalent (the test and the reference products) under fasting and fed conditions, and thus both can be used interchangeably in the clinical setting.
Keywords: flupirtine, D-13223, LC–MS/MS