108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

BRAF 和 MEK 抑制剂联合治疗恶性黑色素瘤患者的疗效和安全性 :一项荟萃分析
Authors Chen P, Chen FC, Zhou BH
Received 27 July 2017
Accepted for publication 6 October 2017
Published 13 November 2017 Volume 2017:10 Pages 5391—5403
DOI https://doi.org/10.2147/OTT.S147438
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
Background: Recent clinical studies have shown that initial therapy with
combined BRAF and mitogen-activated
extracellular signal-regulated kinase (MEK) inhibition is more effective in
metastatic melanoma than single-agent BRAF inhibitors.
However, the response rates with single-agent BRAF are
low. Thus, the objective of this study was to conduct a meta-analysis of
randomized controlled trials to compare the efficacy and adverse events risk
between monotherapy and combination therapy.
Materials and methods: Searches were made in PubMed and EMBASE
electronic databases and conference abstracts published by the American Society
of Clinical Oncology from 2000 to 2017. Outcomes included overall response,
progression-free survival, and overall survival, as well as the incidence rate
of adverse events.
Results: Eight trials comprising 2,664 patients were included
in the meta-analysis. Patients with combined therapies showed superior results
compared to those with BRAF inhibitors
alone for the following: overall response rate (combined relative risk
[RR] =1.34, 95% confidence interval [95% CI]: 1.24–1.45, P <0.00001), progression-free
survival (combined hazards ratio [HR] =0.58, 95% CI: 0.52–0.64, P <0.00001), and overall
survival rate (combined HR =0.70, 95% CI: 0.62–0.80, P <0.00001). Patients with
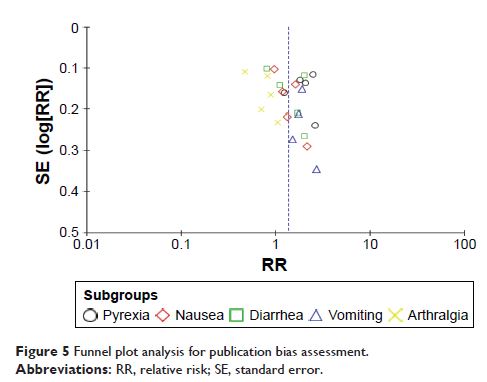
combination therapies had higher incidence of adverse events including pyrexia
(combined RR =2.00, 95% CI: 1.40–2.84), nausea (combined RR =1.41,
95% CI: 1.03–1.94), diarrhea (combined RR =1.50, 95% CI: 1.08–2.06), and
vomiting (combined RR =1.87, 95% CI: 01.52–2.31) compared to those
with BRAF inhibitors alone.
Conclusion: These data suggested that the combined BRAF and MEK inhibition was
associated with a significant improvement in overall response, progression-free
survival, and overall survival, but increased the incidence of adverse events
among the patients with BRAF V600-mutated
metastatic melanoma. Further large-scale, high-quality, placebo-controlled,
double-blind trials are needed to confirm this conclusion.
Keywords: efficacy,
safety, melanoma, meta-analysis, BRAF inhibition,
MEK inhibition