108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

用 PARP 抑制剂治疗的癌症患者的严重血液毒性风险:随机对照试验的荟萃分析
Authors Zhou JX, Feng LJ, Zhang X
Received 30 July 2017
Accepted for publication 18 September 2017
Published 13 October 2017 Volume 2017:11 Pages 3009—3017
DOI https://doi.org/10.2147/DDDT.S147726
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Purpose: Hematologic toxicities, including neutropenia, thrombocytopenia, and
anemia, are major adverse effects of PARP inhibitors (PARPis), but the
incidence rate and overall risk has not been systematically studied. Therefore,
we conducted a meta-analysis of published clinical trials to investigate the
incidence and relative risks (RRs) of severe (high-grade) hematologic events in
cancer patients treated with PARPis.
Methods: PubMed, Embase, and oncology conference proceedings
were searched for relevant studies. Eligible studies were Phase II and III
randomized controlled trials (RCTs) of PARPis in cancer patients with adequate
safety data on hematologic toxicities. The summary incidence, RRs, and 95%
confidence intervals (CIs) were calculated.
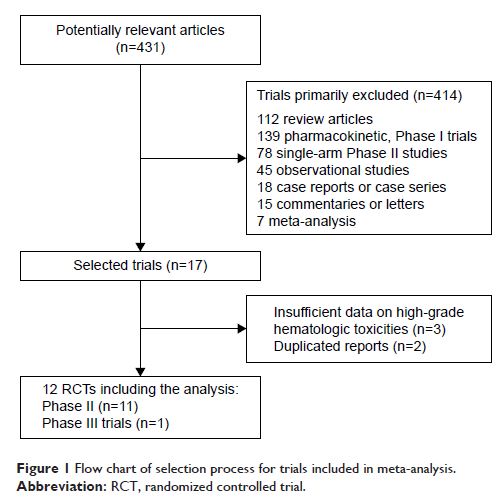
Results: A total of 2,479 patients from 12 RCTs revealed that
the incidence of PARPi-associated severe hematologic toxicities was,
respectively: neutropenia: 32.9% (95% CI, 20.5%–48.3%); thrombocytopenia: 15.9%
(95% CI, 9.5%–25.4%), and anemia: 9.1% (95% CI, 5.1%–15.7%). Olaparib was
associated with an increased risk of severe neutropenia. Veliparib was
associated with an increased risk of severe neutropenia and thrombocytopenia.
Niraparib was associated with an increased risk of severe thrombocytopenia,
anemia, and neutropenia. When stratified by combination therapy, significantly
increased risk of hematologic toxicities was observed for patients treated with
PARPis monotherapy and PARPis combined with single-agent chemotherapy.
Conclusion: Treatment with PARPis olaparib, veliparib, and
niraparib is associated with a significant increase in the risk of hematologic
toxicities in cancer patients, and frequent clinical monitoring should be
emphasized when managing these PARPis.
Keywords: hematologic
toxicities, PARP inhibitors, cancer, meta-analysis, RCTs