108985
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

内吞抑制剂和 NF-kB 信号通路对叶酸 (folate) 偶联纳米颗粒在大鼠 Kupffer 细胞中内吞作用的影响
Authors Tang H, Chen H, Jia Y, Liu X, Han Z, Wang A, Liu Q, Li X, Feng X
Received 9 May 2017
Accepted for publication 13 July 2017
Published 18 September 2017 Volume 2017:12 Pages 6937—6947
DOI https://doi.org/10.2147/IJN.S141407
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Thiruganesh Ramasamy
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Abstract: The regular accumulation of nanoparticles in the liver makes them
hepatotoxic and decreases the circulation time, thus reducing their therapeutic
effect. Resolving this problem will be significant in improving bioavailability
and reducing side effects. In this study, we reduced the phagocytosis of
epirubicin (EPI)-loaded folic acid-conjugated pullulan acetate (FPA/EPI)
nanoparticles by Kupffer cells (KCs) through internalization and nuclear factor
kappa B (NF-kB) signal pathway inhibitors, thus allowing development of FPA/EPI
nanoparticles as a nanodrug delivery system (NDDS) based on our previous study.
FPA/EPI nanoparticles were prepared by the dialysis method. Rat KCs were
preincubated with the following individual or compound inhibitors:
chlorpromazine (CPZ), nystatin (NY), colchicine (Col), amiloride (AMR), and
pyrrolidine dithiocarbamate (PDTC). Dose- and time-dependent cellular uptake
effects of inhibitors on FPA/EPI nanoparticles were determined through
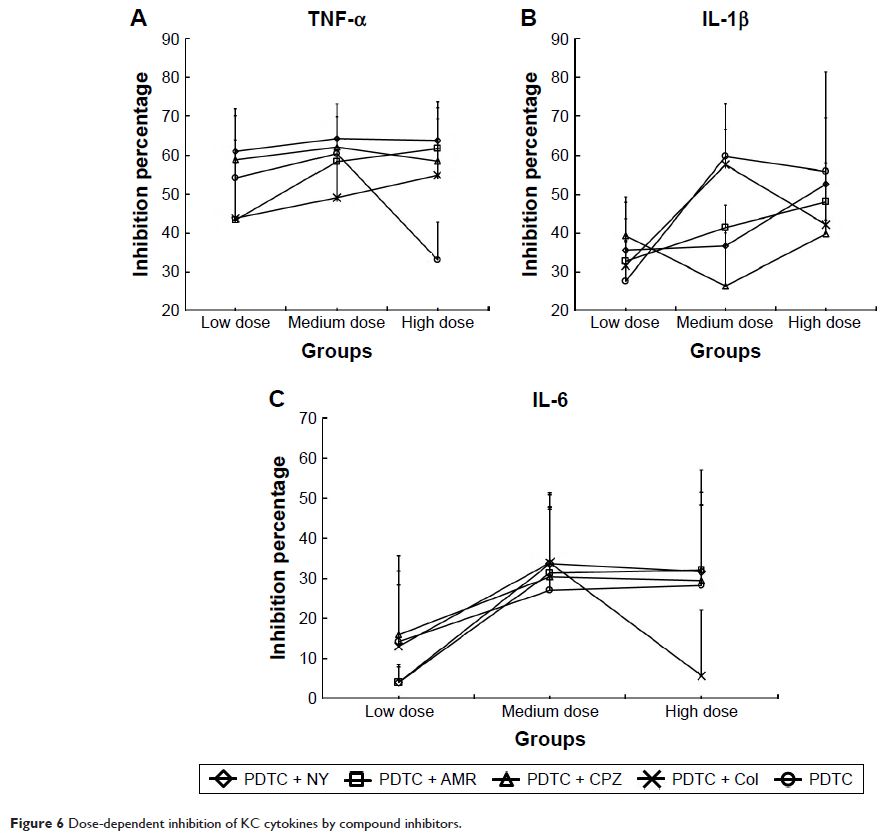
fluorometry. The cytokine levels of tumor necrosis factor alpha (TNF-α),
interleukin-1 beta (IL-1β), and IL-6 were tested in culture supernatants by
bead-based multiplex flow cytometry. The uptake study demonstrated that
inhibitors had an obvious inhibitory effect (P <0.05 or P <0.01), with NY, AMR and Col
all showing time-dependent inhibitory effects. PDTC + NY had the strongest
inhibitory effect, with an uptake rate of 14.62%. The levels of the three
proinflammatory cytokines were changed significantly by the compound
inhibitors. TNF-α was significantly inhibited (P <0.05
or P <0.01), but IL-1β and IL-6
showed smaller decreases. These results suggested that clathrin- and
caveolae-mediated endocytosis were the main routes via which nanoparticles
entered KCs and that the NF-kB signal pathway was very important too. In
summary, multiple mechanisms, including clathrin- and caveolae-mediated
endocytosis, contribute to cytokine production in macrophages following
exposure to folic acid-conjugated pullulan acetate nanoparticles. Thus, the
endocytosis inhibition strategy has great potential for improving therapy and
reducing toxicity of an NDDS in the treatment of cancer.
Keywords: nanodrug
delivery system, Kupffer cells, endocytosis inhibitor, folate-conjugated
pullulan acetate, NF-kB signal pathway