109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD
Authors Watz H, Troosters T, Beeh KM, Garcia-Aymerich J, Paggiaro P, Molins E, Notari M, Zapata A, Jarreta D, Garcia Gil E
Received 8 June 2017
Accepted for publication 8 August 2017
Published 24 August 2017 Volume 2017:12 Pages 2545—2558
DOI https://doi.org/10.2147/COPD.S143488
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Charles Downs
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
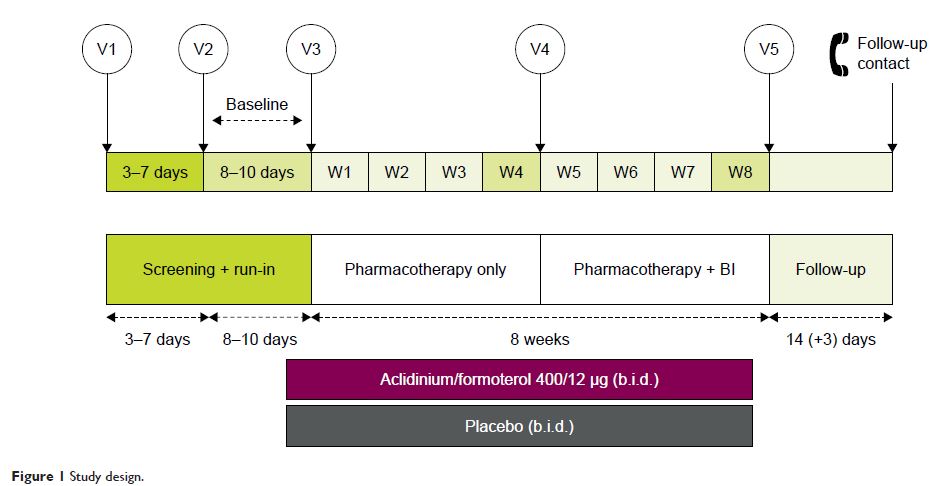
Abstract: The Phase IV, 8-week, randomized, double-blind, placebo-controlled
ACTIVATE study (NCT02424344) evaluated the effect of aclidinium/formoterol
(AB/FF) 400/12 µg twice daily on lung hyperinflation, exercise capacity,
and physical activity in patients with moderate-to-severe COPD. Patients
received AB/FF (n=134) or placebo (n=133) (1:1) via the Genuair™/Pressair® dry powder inhaler for 8 weeks. From Weeks 5
to 8, all patients participated in behavioral intervention (BI; daily messages
providing step goals). The primary end point was trough functional residual
capacity (FRC) at Week 4. Exercise endurance time and physical activity
were assessed at Week 4 (pharmacotherapy only) and at Week 8 (8 weeks
of pharmacotherapy plus 4 weeks of BI). Other end points included post-dose
FRC, residual volume, and inspiratory capacity (IC) at rest and during
exercise. After 4 weeks, trough FRC improved with AB/FF versus placebo but did
not reach significance (125 mL; P =0.0690). However,
post-dose FRC, residual volume, and IC at rest improved significantly with
AB/FF at Week 4 versus placebo (all P <0.0001).
AB/FF significantly improved exercise endurance time and IC at isotime versus
placebo at Week 4 (P <0.01 and P <0.0001, respectively)
and Week 8 (P <0.05 and P <0.0001, respectively).
AB/FF achieved higher step counts (P <0.01) with fewer
inactive patients (P <0.0001) at
Week 4 versus placebo. Following BI, AB/FF maintained improvements in
physical activity at Week 8 and nonsignificant improvements were observed
with placebo. AB/FF 400/12 µg demonstrated improvements in lung hyperinflation,
exercise capacity, and physical activity versus placebo that were maintained
following the addition of BI. A 4-week period of BI might be too short to
augment the improvements of physical activity observed with AB/FF.
Keywords: COPD,
hyperinflation, aclidinium, formoterol, exercise capacity, physical activity