109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

母体暴露于纳米二氧化钛会抑制小鼠胚胎发育
Authors Hong F, Zhou Y, Zhao X, Sheng L, Wang L
Received 9 June 2017
Accepted for publication 8 August 2017
Published 24 August 2017 Volume 2017:12 Pages 6197—6204
DOI https://doi.org/10.2147/IJN.S143598
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Abstract: Although
nanoscale titanium dioxide (nano-TiO2) has been extensively used in industrial food applications and daily
products for pregnant women, infants, and children, its potential toxicity on
fetal development has been rarely studied. The main objective of this
investigation was to establish the effects of maternal exposure of nano-TiO2 on developing embryos. Female imprinting control region mice were
orally administered nano-TiO2 from gestational day 0 to 17. Our findings showed that Ti
concentrations in maternal serum, placenta, and fetus were increased in
nano-TiO2-exposed mice when
compared to controls, which resulted in reductions in the contents of calcium
and zinc in maternal serum, placenta, and fetus, maternal weight gain,
placental weight, fetal weight, number of live fetuses, and fetal crown–rump
length as well as cauda length, and caused an increase in the number of both
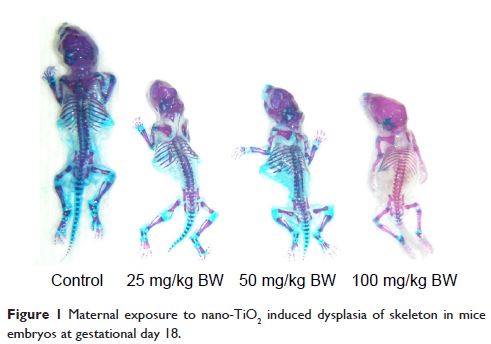
dead fetuses and resorptions. Furthermore, maternal nano-TiO2 exposure inhibited development of the fetal skeleton, suggesting a
significant absence of cartilage, reduced or absent ossification, and an
increase in the number of fetuses with dysplasia, including exencephaly, spina
bifida, coiled tail, scoliosis, rib absence, and sternum absence. These
findings indicated that nano-TiO2 can cross the blood–fetal barrier and placental barrier, thereby
delaying the development of fetal mice and inducing skeletal malformation.
These factors may be associated with reductions in both calcium and zinc in
maternal serum and the fetus, and both the placenta and embryos may be major
targets of developmental toxicity following maternal exposure to nano-TiO2 during the prenatal period. Therefore, the application of nano-TiO2 should be carried out with caution.
Keywords: nanosized titanium dioxide, maternal exposure, embryonic toxicity,
skeleton developmental suppression