109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

铬的致癌性和化学预防:一个扼要更新
Authors Wang Y, Su H, Gu Y, Song X, Zhao J
Received 9 April 2017
Accepted for publication 13 June 2017
Published 16 August 2017 Volume 2017:10 Pages 4065—4079
DOI https://doi.org/10.2147/OTT.S139262
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Ashok Kumar Pandurangan
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
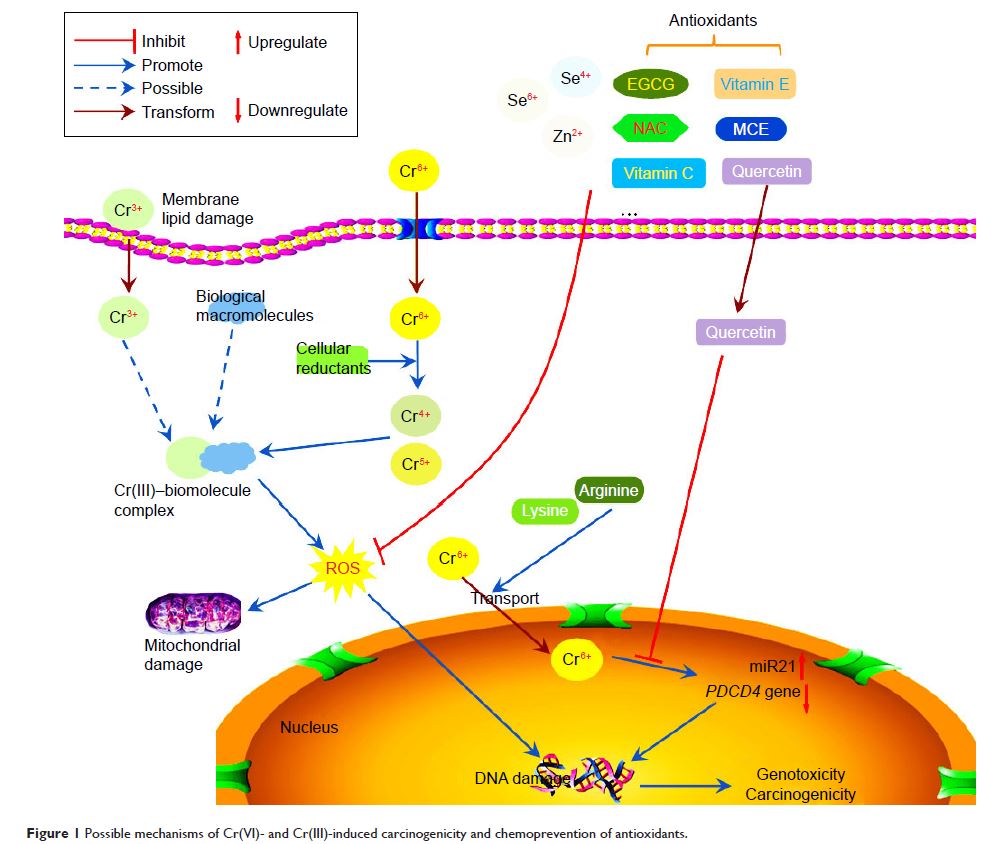
Abstract: Chromium has two main
valence states: hexavalent chromium (Cr[VI]) and trivalent chromium (Cr[III]).
Cr(VI), a well-established human carcinogen, can enter cells by way of a
sulfate/phosphate anion-transport system, and then be reduced to lower-valence
intermediates consisting of pentavalent chromium (Cr[V]), tetravalent chromium
(Cr[IV]) or Cr(III) via cellular reductants. These intermediates may directly
or indirectly result in DNA damage or DNA–protein cross-links. Although Cr(III)
complexes cannot pass easily through cell membranes, they have the ability to
accumulate around cells to induce cell-surface morphological alteration and
result in cell-membrane lipid injuries via disruption of cellular functions and
integrity, and finally to cause DNA damage. In recent years, more research,
including in vitro, in vivo, and epidemiological studies, has been
conducted to evaluate the genotoxicity/carcinogenicity induced by Cr(VI) and/or
Cr(III) compounds. At the same time, various therapeutic agents, especially
antioxidants, have been explored through in vitro and in vivo studies for
preventing chromium-induced genotoxicity/carcinogenesis. This review aims to
provide a brief update on the carcinogenicity of Cr(VI) and Cr(III) and
chemoprevention with different antioxidants.
Keywords: hexavalent
chromium, Cr(VI), trivalent chromium, Cr(III), genotoxicity, carcinogenicity,
chemoprevention, antioxidant