109451
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

SHP2 过表达增强了体外和体内卵巢癌的侵袭和转移
Authors Hu ZQ, Li J, Gao Q, Wei S, Yang B
Received 4 April 2017
Accepted for publication 30 May 2017
Published 2 August 2017 Volume 2017:10 Pages 3881—3891
DOI https://doi.org/10.2147/OTT.S138833
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Ashok Kumar Pandurangan
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Purpose: SHP2
has roles in a variety of signal transduction pathways and in many important
cellular processes, including proliferation, differentiation, movement
regulation, and apoptosis. In addition, SHP2 expression is closely associated
with multiple types of malignancies. In this study, we examined the role of
SHP2 in epithelial ovarian cancer.
Patients and
methods: SHP2 expression in cancer and normal
ovarian tissue specimens was evaluated by immunohistochemical staining and
Western blot analyses. The correlation between the SHP2 expression level and
clinicopathological features was analyzed. The role of SHP2 in epithelial
ovarian cancer was evaluated by assessing SHP2 expression patterns in vitro and
in vivo, and activation of the PI3K/AKT pathway was examined.
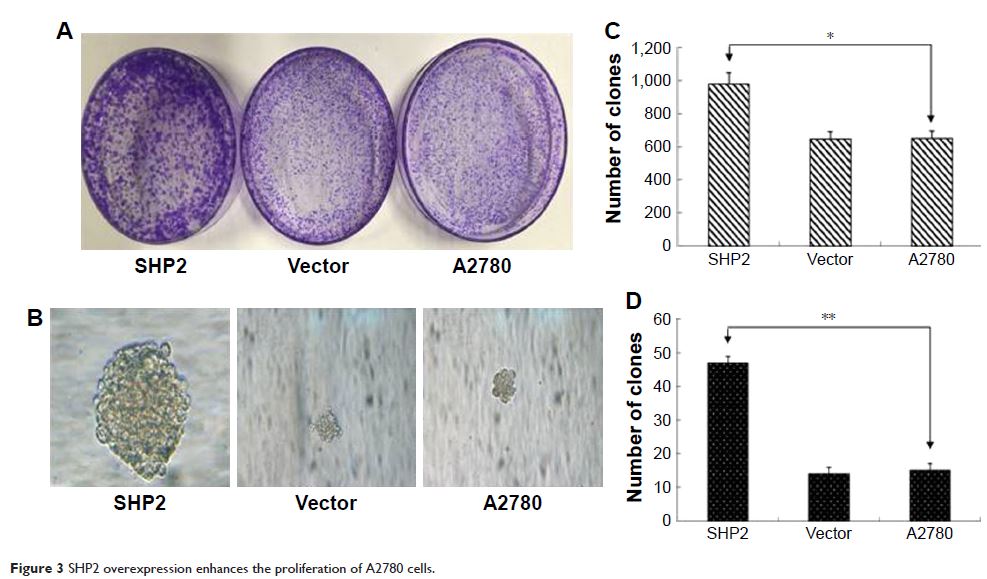
Results: SHP2 is expressed at higher levels in ovarian cancer tissues than in
normal ovarian tissues and in an ovarian cancer cell line than in a normal
ovarian cell line. On the basis of these findings, SHP2 is overexpressed in
ovarian cancer both in vitro and in vivo. In addition, SHP2 overexpression is
associated with tumor stage and differentiation, enhanced cell proliferation
and invasion, and tumorigenesis and metastasis.
Conclusion: SHP2 overexpression enhances ovarian tumor proliferation and
invasion by activating the PI3K-AKT axis, indicating that SHP2 potentially
plays a direct role in the pathogenesis of ovarian epithelial cell cancer.
These novel findings provide key insights that are applicable to basic cancer
research and to the prevention and treatment of cancer.
Keywords: ovarian tumor, SHP2, overexpression, proliferation, invasion,
metastasis